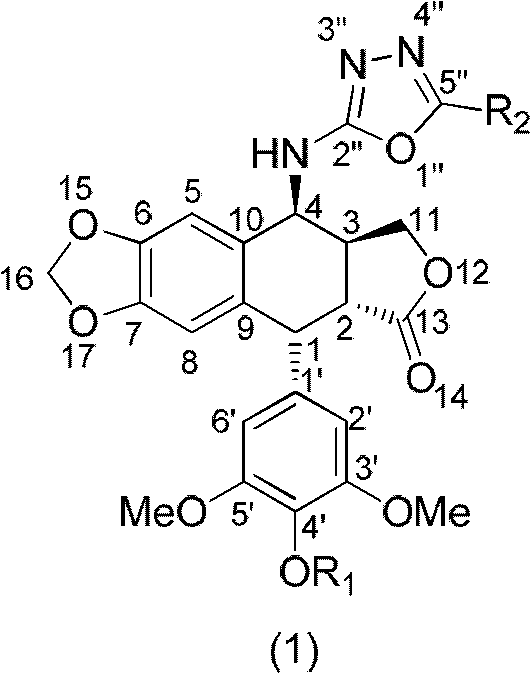
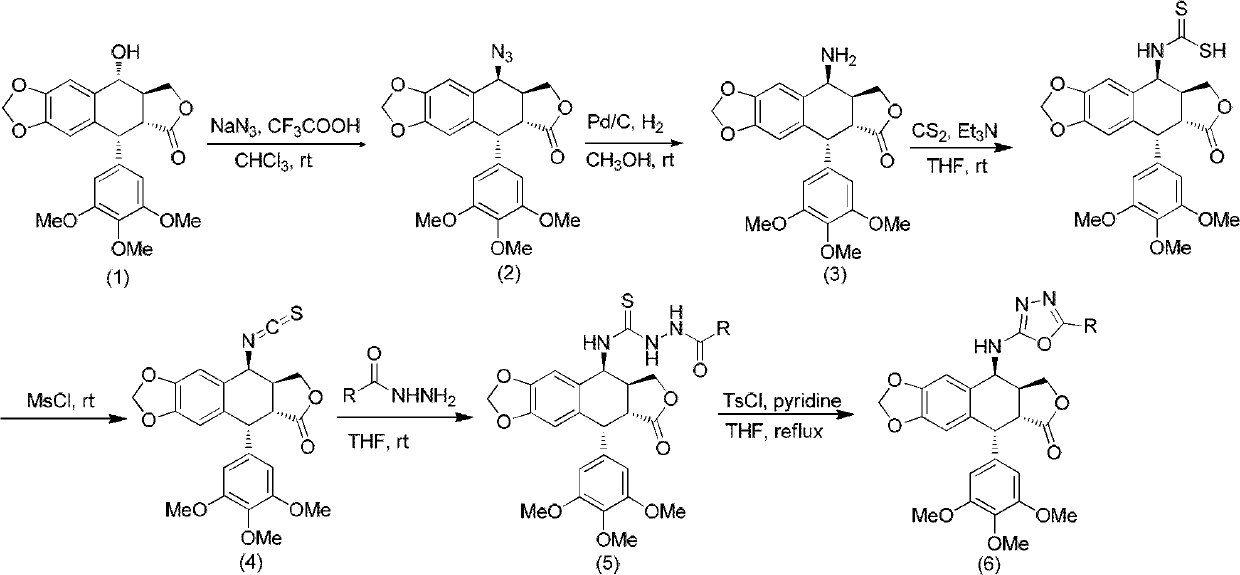
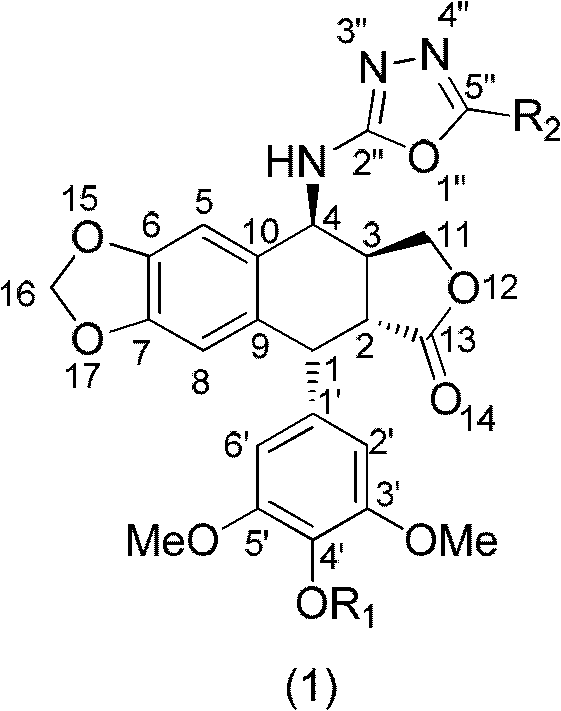
4-amino oxadiazole epipodophyllotoxin derivative and preparation method and application thereof
A technology of aminooxadiazole epipodophyllotoxin, which is applied in the application field of 4-aminooxadiazole epipodophyllotoxin derivatives, can solve the problems of poor water solubility and poor oral effect, and achieve good cytotoxicity, Improvement of water solubility, reduction of normal cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Compound 2 preparation of
[0029] Add podophyllotoxin (830mg, 2mmol), sodium azide (650mg, 10mmol), chloroform 20mL, and trifluoroacetic acid 4mL sequentially in a 100mL round-bottom flask under ice-bath conditions, and stir at room temperature for 6h. TLC detects that the reaction is over, adding saturated sodium carbonate solution (20mL), separating the organic layer, washing the organic layer with water (20mL) and saturated brine (20mL) respectively, and extracting the aqueous layer with ethyl acetate (2×20mL) Wash with water (20mL) and saturated brine (20mL), combine the organic layers, wash with anhydrous MgSO 4 After drying, the desiccant was filtered off, the filtrate was evaporated to dryness under reduced pressure, and separated by silica gel column chromatography (petroleum ether: ethyl acetate = 3:1 (volume ratio)) to obtain the target product. 1 H NMR (CDCl 3 , 500MHz): δ2.91-2.98 (1H, m, 3-H), 3.18 (1H, dd, J=5.0Hz, 2-H), 3.74 (6H, s, 3′, 5′-...
Embodiment 2
[0030] Example 2 Compound 3 preparation of
[0031] Azidopodophyllotoxin (826 mg, 2 mmol), 15 ml of methanol, and 80 mg of 10% Pd / C were sequentially added to a 50 mL round bottom flask, and stirred overnight at room temperature under a hydrogen atmosphere. After the reaction was detected by TLC, the Pd / C was filtered off, the filtrate was evaporated to dryness under reduced pressure, and separated by silica gel column chromatography (petroleum ether: ethyl acetate = 1:2 (volume ratio)), and the target product was obtained. 1 HNMR (CDCl 3, 500MHz): δ2.81-2.88 (1H, m, 3-H), 3.30 (1H, dd, J=5.5Hz, 2-H), 3.74 (6H, s, 3′, 5′-OCH 3 ), 3.79 (3H, s, 4'-OCH 3 ), 4.21 (1H, d, J = 4.0Hz, 4-H), 4.27-4.33 (2H, m, 11-H), 4.56 (1H, d, J = 5.0Hz, 1-H), 5.96 (1H , s, OCHO), 5.97 (1H, s, OCHO), 6.30 (2H, s, 2′, 6′-H), 6.49 (1H, s, 8-H), 6.82 (1H, s, 5-H ); 13 C NMR (CDCl 3 , 500MHz): δ38.15 (3-C), 40.20 (2-C), 43.98 (1-C), 48.95 (4'-OCH 3 ), 56.28 (3',5'-OCH 3 ), 60.75(4-C), 68.18(11...
Embodiment 3
[0032] Example 3 Compound 4 preparation of
[0033] Add aminoepipodophyllotoxin (206mg, 0.5mmol), anhydrous THF 4mL, Et 3 N (3.5equiv, 0.24mL), cooled to 0°C, dissolved CS in 1mL of anhydrous THF 2 (0.05mL) was added slowly, and the ice bath was removed after the dropwise addition, and stirred at room temperature for 1.5h. After cooling to 0°C, MsCl (0.043 mL, 1.1 equiv) was added, the ice bath was removed after the addition, and the mixture was stirred at room temperature for 1 h. After the reaction was detected by TLC, 20 mL of ethyl acetate was added, washed with 1N HCl (2×20 mL), water (20 mL), saturated brine (20 mL), and washed with anhydrous MgSO 4 Dry and filter to remove the desiccant, evaporate the filtrate to dryness under reduced pressure, and separate by silica gel column chromatography (petroleum ether: ethyl acetate = 3:1 (volume ratio)) to obtain the target product. MS (ESI, m / z): [M+Na] + 478.1; 1 H NMR (CDCl 3 , 500MHz): δ2.88-2.95 (1H, m, 3-H), 3.13 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com