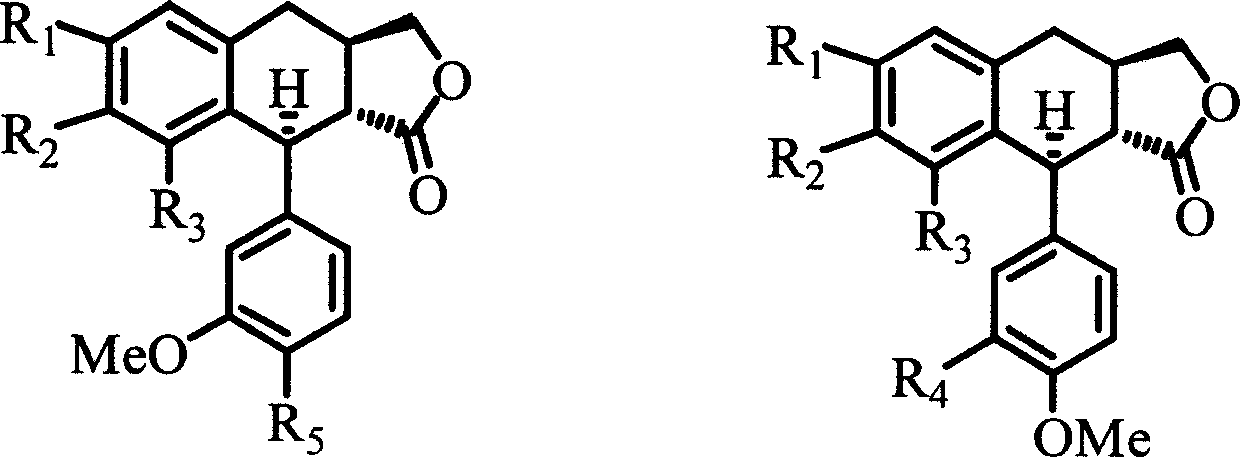
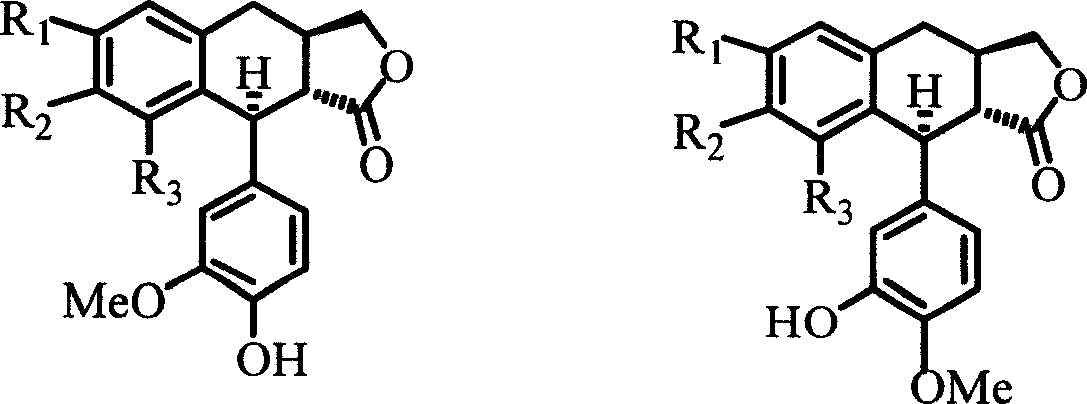
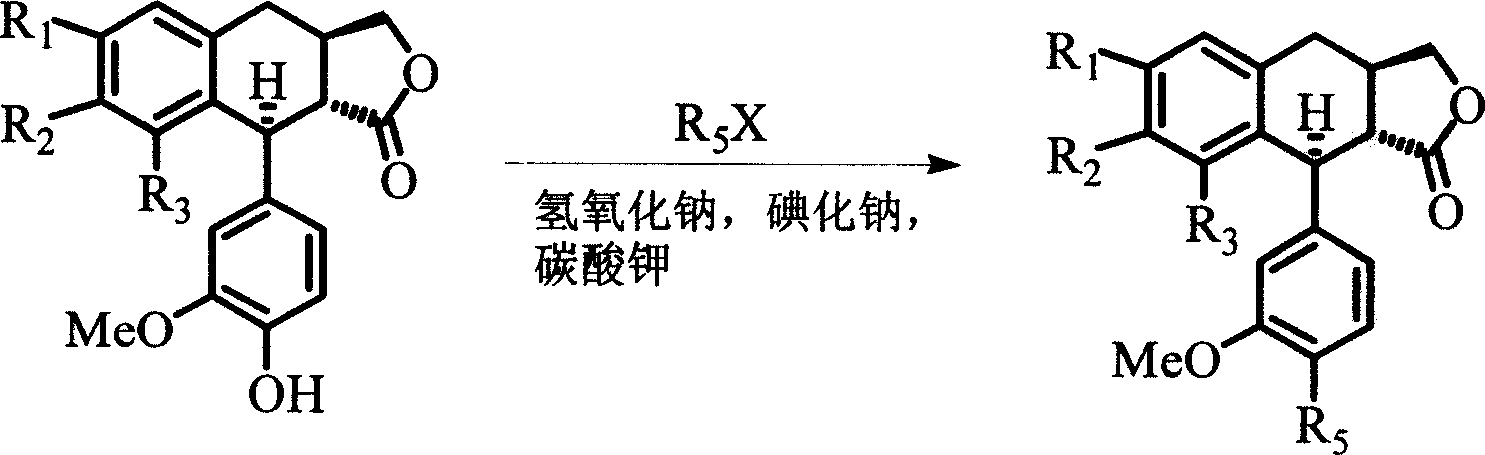
4-deoxyisopodophyllotoxin derivatives, preparation and medicinal uses thereof
A technology for deoxygenation of alien ghosts and toxins, which can be used in the fields of medicinal chemistry and pharmacology to solve problems such as bone marrow suppression and poor water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Compound III-4[(3S,4R)-3-(3-methoxy-4-methoxymethoxy-α-hydroxybenzyl)-4-(3,4,5-trimethyl Oxybenzyl)dihydrofuran-2-one] preparation:
[0061]
[0062] Under nitrogen protection, 0.34 ml (2.4 mmol) of diisopropylamine was dissolved in 20 ml of dry tetrahydrofuran (THF), cooled to -78°C, and 1.5 ml (2.4 mmol), then 10 milliliters of THF solution with 532 mg (2.0 mmol) of 4-(3,4,5-trimethoxybenzyl)-4,5-dihydrofuran-2-one was slowly added dropwise to the reaction In the system, 8 milliliters of THF solutions that are dissolved with 392 mg (2.0 mmol) of 3-methoxy-4-methoxybenzaldehyde are slowly added dropwise into the reaction flask, and after the addition is completed, it is naturally warmed up to At room temperature, saturated ammonium chloride solution (20 ml) was added, the organic layer was separated, the aqueous layer was extracted with ethyl acetate (15 ml×3), the organic phases were combined, and dried over anhydrous sodium sulfate. After filtration, ...
Embodiment 2
[0071] II-1a compound [C(7″)-αH]: Rf (ethyl acetate / petroleum ether: 1 / 1): 0.30; 1H NMR (400MHz, CDCl3): δ2.30-2.41(m, 2H, H- 7), 2.65(m, 1H, Hz, H-3), 2.86(m, 1H, H-4), 2.97(brs, 1H, OH-7″β), 3.50(s, 3H, OCH3), 3.69 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 4.13 (brs, 1H, H-5β), 4.28 (t, 1H, J=8.4, 8.4Hz, H-5α), 5.22 (s, 2H, OCH20), 5.29 (brs, 1H, H-7″α), 6.36 (d, 1H, J=1.6Hz, H-2'), 6.42 (dd, 1H, J=8.0, 2.0Hz, H-6'), 6.65(d, 1H, J=8.0Hz, H-5'), 6.81(d, 1H, J=8.0Hz, H-5"), 6.90(d , 1H, J=2.0Hz, H-2″), 7.03 (dd, 1H, J=8.0, 2.0Hz, H-6″).
[0072] III-1b compound [C(7″)-βH]: Rf (ethyl acetate / petroleum ether: 1 / 1): 0.30; 1HNMR (400MHz, CDCl3): δ2.20(t, 2H, J=9.2, 7.2 Hz, H-7), 2.52(m, 1H, H-4), 2.63(t, 1H, J=4.4, 3.6Hz, H-3), 3.49(s, 3H, OCH3), 3.69(s, 3H , OCH3), 3.82(s, 3H, OCH3), 3.87(s, 3H, OCH3), 3.91(dd, 1H, J=5.2, 2.8Hz, H-5β), 4.09(t, 1H, J=8.4, 8.4Hz, H-5α), 4.81(d, 1H, J=8.0Hz, H-7″β), 5.26(s, 2H, OCH2O), 6.38(d, 1H, J=2.0Hz, H-2' ), 6.46 (dd, 1H, J=...
Embodiment 3
[0074] III-2a compound [C(7″)-αH]: Rf (ethyl acetate / petroleum ether: 1 / 1): 0.30; 1H NMR (400MHz, CDCl3): δ2.44 (dd, 1H, J=14.4, 8.8Hz, H-7α), 2.30(dd, 1H, J=10.4, 7.2Hz, H-7β), 2.64(dd, 1H, J=12.4, 3.6Hz, H-3), 2.82(m, 1H, H-4), 3.03(brs, 1H, OH-7″β), 3.48(s, 3H, OCH3), 3.78(s, 3H, OCH3), 3.80(s, 3H, OCH3), 3.83(s, 3H , OCH3), 4.07(brs, 1H, H-5β), 4.31(t, 1H, J=8.4, 8.0Hz, H-5α), 5.22(s, 2H, OCH20), 5.28(d, 1H, J= 5.6Hz, H-7″α), 6.36(d, 1H, J=8.0Hz, H-6’), 6.42(s, 1H, H-2’), 6.65(d, 1H, J=8.0Hz, H-5'), 6.80 (d, 2H, J=8.0 Hz, H-2", 5"), 7.08 (d, 1H, J=8.0 Hz, H-6").
[0075] III-2b compound [C(7″)-βH]: Rf (ethyl acetate / petroleum ether: 1 / 1): 0.30; 1H NMR (400MHz, CDCl3): δ2.21(t, 2H, J=9.2, 4.4Hz, H-7), 2.50(m, 1H, H-4), 2.61(t, 1H, J=4.4, 3.6Hz, H-3), 3.49(s, 3H, OCH3), 3.80(s, 3H, OCH3), 3.83(s, 3H, OCH3), 3.89(s, 3H, OCH3), 3.95(dd, 1H, J=11.2, 9.2Hz, H-5β), 4.13(t, 1H, J=9.2 , 8.0Hz, H-5α), 4.84 (d, 1H, J=8.0Hz, H-7″β), 5.21 (s, 2H, OCH2O), 6.38 (d, 1H, J=8.0Hz, H-6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com