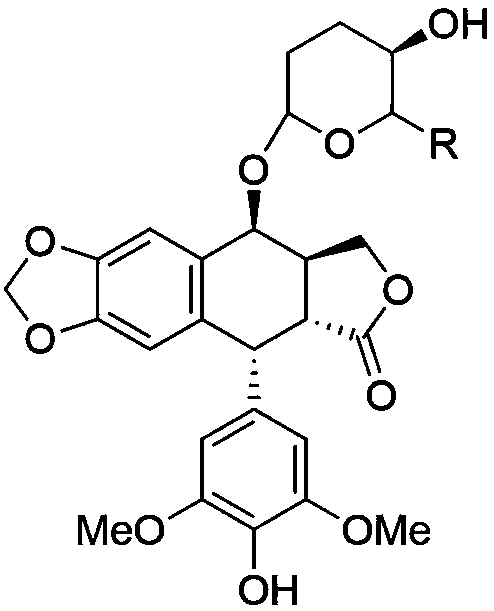
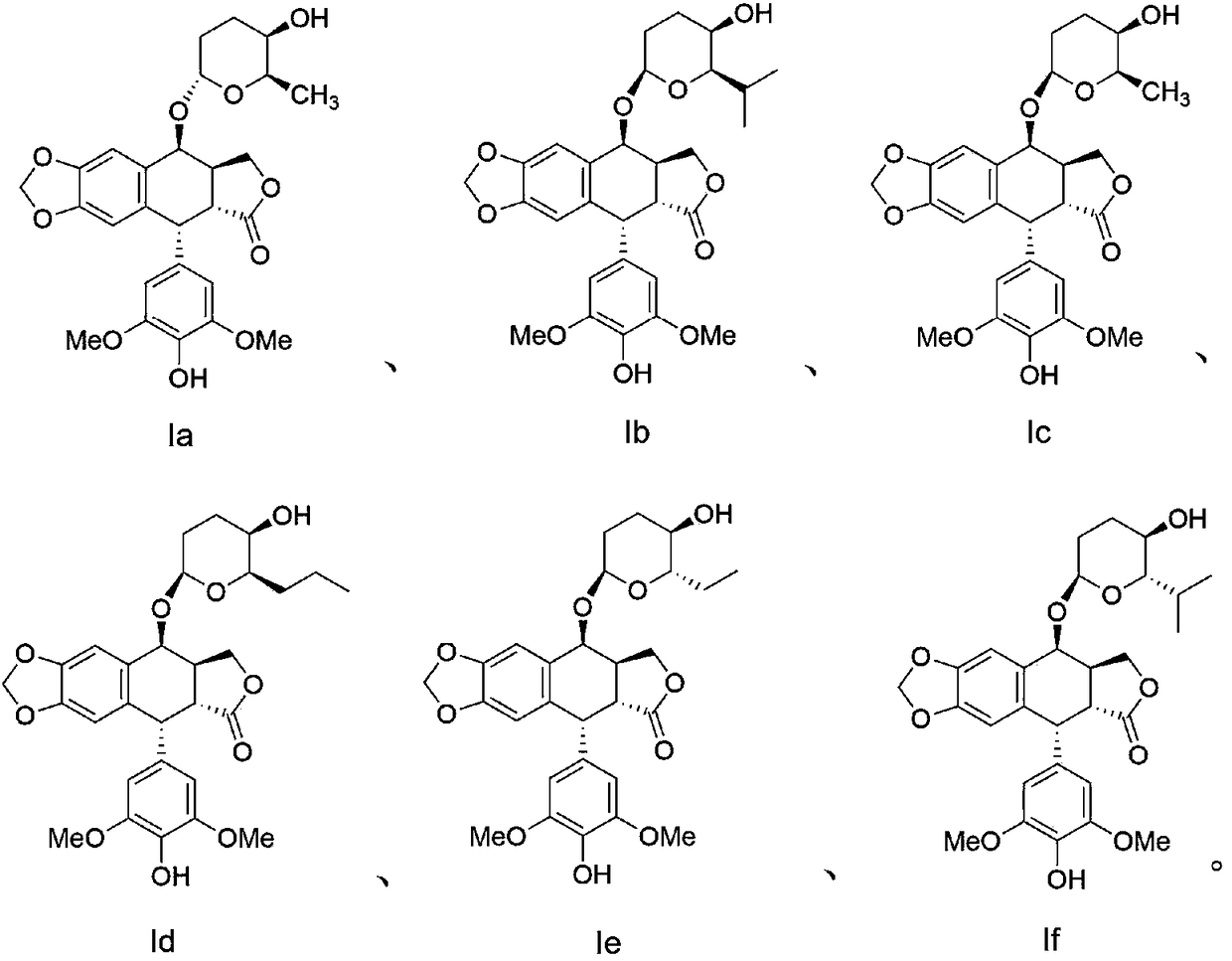
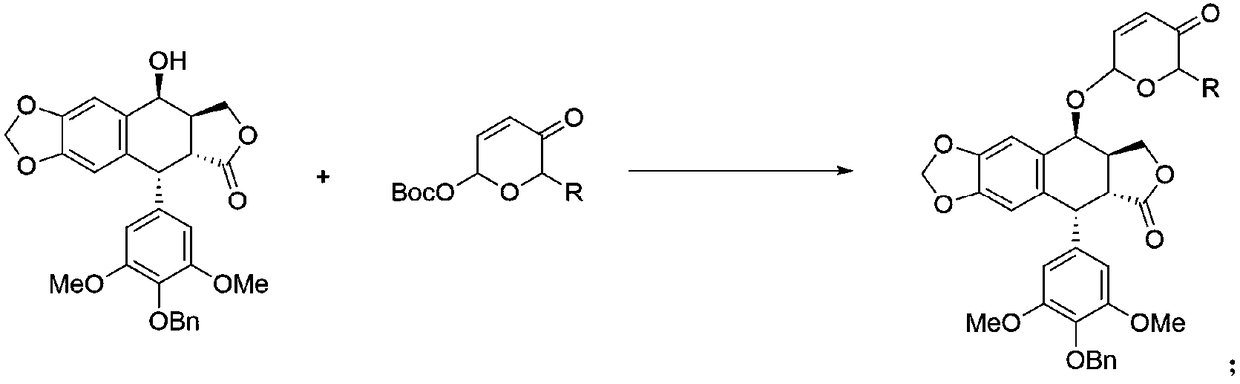
2,3,6-trideoxyglycosyl demethylepipodophyllotoxin compound as well as preparation method and application thereof
A technology for removing epipodophyllin and trideoxyglycosyl, which is applied in the fields of medicinal chemistry and pharmacology, and can solve the problems of easy metabolic inactivation, unsatisfactory therapeutic effect, and poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 (2R, 3R, 6R)-3,6-dihydro-2-methyl-6-norepipodophyllotoxin-2H-pyran-3-ol (Ia)
[0034] Add n-butyl ((2S,6R)-6-methyl-5-oxo-5,6-dihydro-2H-pyran-2-yl)carbonate (912mg, 4mmol) in a 50mL round bottom flask and norepipodophyllotoxin (2.2g, 4.4mmol) were dissolved in 20mL of dichloromethane, and then Pd 2 (dba) 3 (91.6mg, 0.1mmol) and PPh 3 (105.0mg, 0.4mmol), nitrogen protection under ice bath for 12h. The end of the reaction was detected by TLC, concentrated, and column chromatography (prtroleum ether:EtOAc=1:1) gave a slightly yellow solid IIIa (1.4g), with a yield of 60%.
[0035] In a 50mL round bottom flask, add IIIa (600mg, 1mmol), CeCl 3 • MeOH (5mL, 0.4eq), sodium borohydride (42mg, 1.1mmol) and 5mL of dichloromethane were mixed and stirred at -78°C for 2.5h. The end of the reaction was detected by TLC, quenched with deionized water, extracted with dichloromethane (50mL×3), dried over anhydrous sodium sulfate, filtered, concentrated, column chromatogra...
Embodiment 2
[0038] The method for reference example 1 prepares Ib-If compound, and the physicochemical data of each compound Ib-If is as follows:
[0039] (2R,3R,6R)-3,6-Dihydro-2-isopropyl-6-norepipodophyllotoxinyl-2H-pyran-3-ol (Ib)
[0040] 1 H NMR (400MHz, DMSO-d 6 ):δ8.24(s,1H,Ar-OH),6.93(s,1H,ArH),6.52(s,1H,ArH),6.21(s,2H,ArH),6.02(d,2H,J= 4.8Hz,CH 2 ), 4.85 (m, 2H, H2, CH), 4.64 (d, 1H, J = 5.6 Hz, CH 2 ),4.52(d,1H,J=5.2Hz,CH 2 ),4.46(m,1H,CH),4.03(m,1H,H6),3.95(m,1H,OH),3.63(s,6H,OCH 3 ),3.33(m,1H,H5),3.20(dd,1H,J 1 =14Hz,J 2 =4.8Hz,CH),2.85(m,1H,CH),2.14(m,1H,CH),1.85(d,1H,J=12.4Hz,H3),1.89(m,3H,H3,H4), 1.08(d, 3H, J=6.8Hz, CH 3 ),0.92(d,3H,J=6.4Hz,CH 3 ). 13 C NMR (100MHz, DMSO-d 6 ): δ 175.0, 147.9, 147.6, 146.8, 135.2, 132.9, 130.6, 130.6, 110.6, 109.9, 109.0, 101.8, 97.3, 77.6, 73.1, 68.2, 66.4, 56.5, 49.1, 483.2, 41.1, 293.4, 38. , 27.7, 20.4, 16.6. HRMS (ESI): m / z calcd for C 29 h 34 o 10 :542.2140; found: 542.2146[M].
[0041] (2R,3R,6R)-3,6-Dihydro-2-meth...
Embodiment 3
[0049] In order to better understand the essence of the present invention, the pharmacological experiment results of the growth inhibitory effect of the 2,3,6-trideoxyglycosyl norepipodophyllotoxin compound of the present invention on five tumor cell lines are as follows: Describe its new application in the field of antitumor drug research. The Pharmacological Examples present partial activity data for representative compounds. It must be noted that the pharmacological examples of the present invention are used to illustrate the present invention rather than limit the present invention. The simple improvements made to the present invention according to the essence of the present invention all belong to the protection scope of the present invention.
[0050] Compounds Ia-If are effective against human lung cancer cells (A549), human liver cancer cells (HepG2), human cervical cancer cells (Hela), human neuroblastoma cells (SH-SY5Y), oral cancer resistant vincristine cells (KB / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com