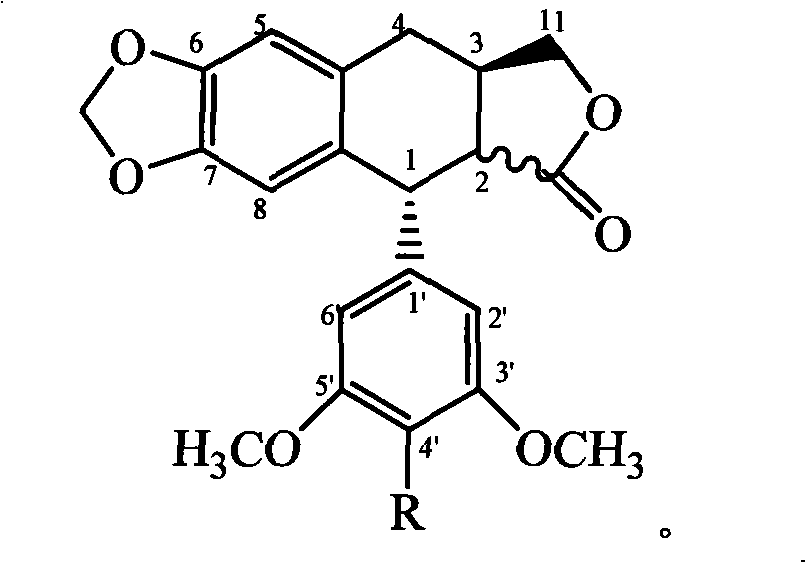
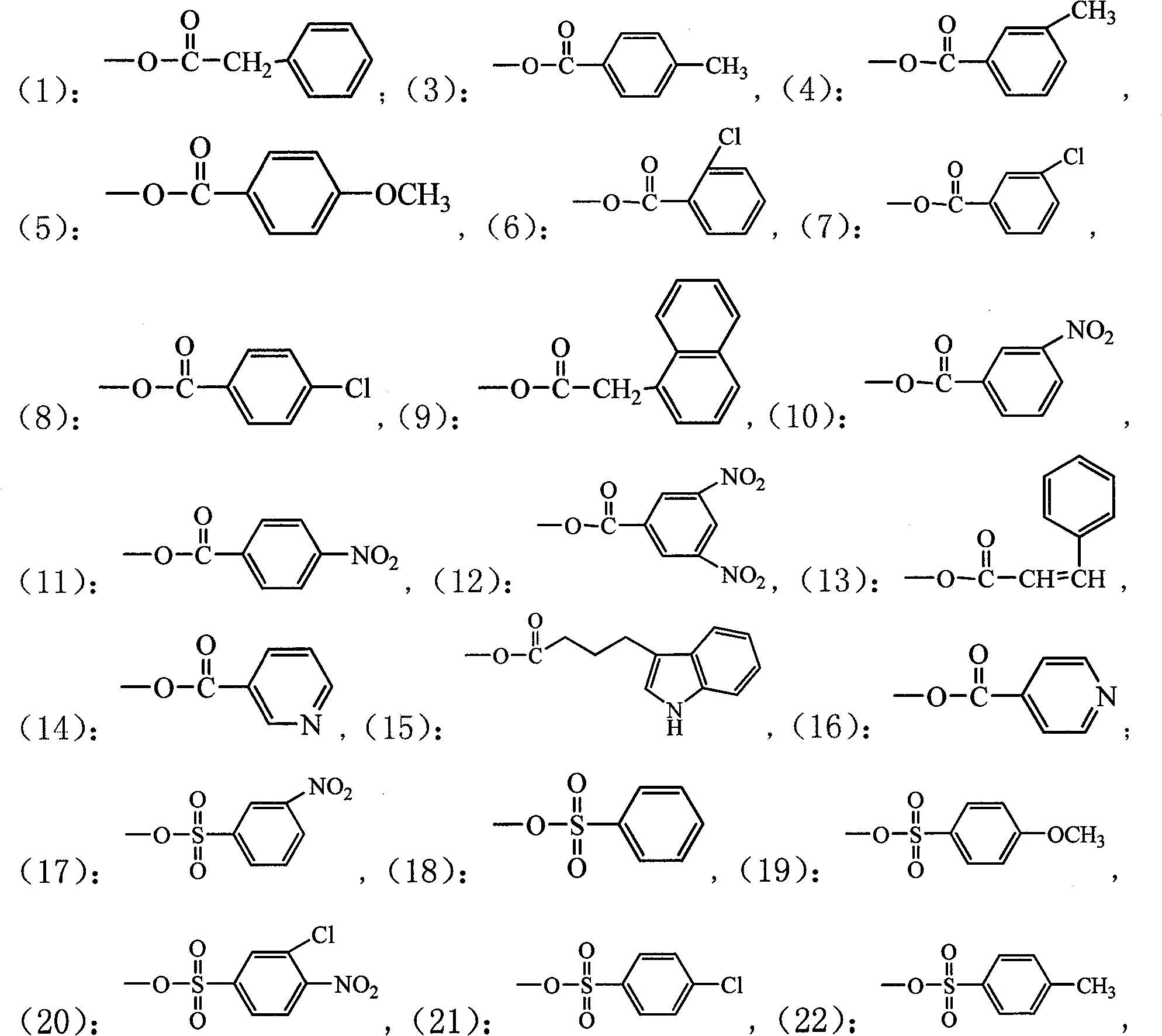4'-podophyllotoxin demethyl deoxidated aromatic ester, substituted benzene sulfonate, ether derivative and use in plant source pesticide preparation
The technology of methyldeoxypodophyllin and aromatic acid ester is applied in the application field of preparing botanical insecticides, which can solve the problems of delayed growth and development, the rate of pupation, the rate of eclosion, and the reduction of egg production, and achieves strong antifeeding and poisoning. active effect
Inactive Publication Date: 2010-09-08
NORTHWEST A & F UNIV
View PDF0 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The antitumor and insecticidal activities of deoxypodophyllotoxin and its derivatives have been reported in literature, such as: [Young-Jae You, Yong Kim, Nguyen-Hai Nam, Byung-Zun Ahn.Antitumor activity of unsaturated fatty acid esters of 4′ -demethyldeoxypodophyllotoxin[J].Bioorganic & Medicinal Chemistry Letters.2003,13:2629-2632.] and [Young-Jae You, Yong Kim, Nguyen-HaiNam, Byung-Zun Ahn, et al.Alkyl and carboxylalkyl esters of 4′- demethyl-4-deoxypodophyllotoxin: synthesis, cytotoxic, and antitumoractivity [J]. European Journal of Medicinal Chemistry.2004, 39: 189-193.] reported the reaction of 4'-deoxypodophyllotoxin with unsaturated and saturated fatty acids The prepared derivatives have good anti-tumor activity in vitro; literature [Zhang Shougang, Hou Huamin, Gao Rong. The toxicity of deoxypodophyllotoxin to Periplaneta americana and the influence of several enzyme systems [J]. Acta Entomology, 2007, 50 (3): 248-252.] reported that deoxypodophyllotoxin has a strong poisonous activity against newly hatched nymphs of Periplaneta americana; Preliminary study on biological activity [J]. Northwest Agricultural Journal, 2005, 14(1): 94-97.] reported that deoxypodophyllotoxin has strong antifeedant activity on armyworm, and also inhibits its growth and development, resulting in growth All stages of development are delayed, pupation rate, eclosion rate, egg production, etc. are all significantly reduced
However, the synthesis of 4'-nordeoxypodophyllotoxin aromatic esters, substituted benzenesulfonate esters and ether derivatives and their insecticidal activity have not been reported.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Login to View More
Abstract
The invention relates to a series of novel 4'-nordeoxypodophyllotoxin aromatic acid esters, substituted benzene sulfonates, and ether derivatives and application thereof in preparing a botanical insecticide. The 4'-nordeoxypodophyllotoxin aromatic acid esters, the substituted benzene sulfonates, and the ether derivatives have a general chemical formula shown at the right. An experiment proves that the 4'-nordeoxypodophyllotoxin aromatic acid esters, the substituted benzene sulfonates, and the ether derivatives are partially higher than podophyllotoxin and deoxypodophyllotoxin, wherein insecticidal activities of certain compounds are all higher than that of a botanical insecticide-toosendanin which has come into the market, and the compounds can be used for preparing the botanical insecticides with high-efficiency and low-toxicity.
Description
technical field The present invention relates to a series of 4'-nordeoxypodophyllotoxin compounds with insecticidal activity, in particular to 4'-nordeoxypodophyllotoxin aromatic esters, substituted benzenesulfonate esters and ether derivatives and the 4' -Application of nordeoxypodophyllotoxin aromatic ester, substituted benzenesulfonate and ether derivatives in the preparation of botanical pesticides. Background technique Deoxypodophyllotoxin is a plant secondary substance isolated from Cypress sativa, which has attracted much attention because of its antitumor activity. In recent years, it has been found that it has antifeedant, poisonous and growth inhibitory effects on various agricultural and forestry pests. The antitumor and insecticidal activities of deoxypodophyllotoxin and its derivatives have been reported in literature, such as: [Young-Jae You, Yong Kim, Nguyen-Hai Nam, Byung-Zun Ahn.Antitumor activity of unsaturated fatty acid esters of 4′ -demethyldeoxypodop...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D493/04A01N43/90A01P7/00
Inventor 徐晖王娟娟
Owner NORTHWEST A & F UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com