Water-soluble 4í»-nor-epipodophyllotoxin derivant and preparation method thereof
A kind of technology of norepiopodophyllin and derivatives, which is applied in the field of derivatives of 4'-norepodophyllotoxin to achieve the effects of simplifying post-processing process, improving reaction yield and improving reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
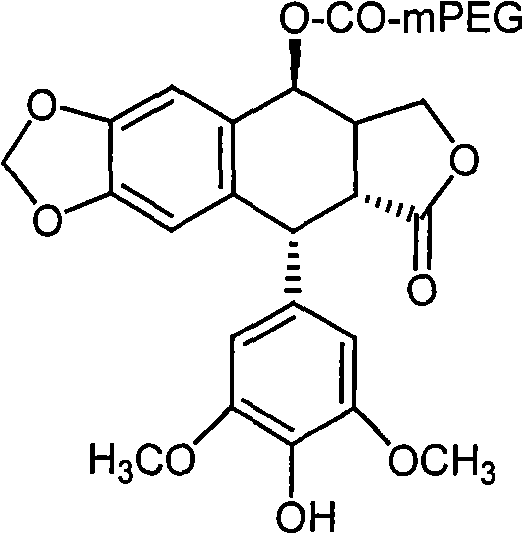
[0024] The preparation route of the present invention is:
[0025] Put 4'-norepipodophyllotoxin and carboxyl monomethoxypolyethylene glycol in a reaction vessel, add dicyclohexylcarbodiimide and dimethylaminopyridine to mix the reactants evenly, and catalyze After 30 minutes of reaction, the 4'-norepipodophyllotoxin prodrug can be prepared.
Embodiment 1
[0027] In a 25ml Erlenmeyer flask, add 1.00g (0.5mmol) mPEG-COOH 2000, 0.30g (0.75mmol) 4'-norepipodophyllotoxin, 0.21g (1.0mmol) dicyclohexylcarbodiimide and 0.12 g (1.0mmol) p-N, N-dimethylaminopyridine, fully shaken to mix the reactants evenly, then placed in a Whirlpool microwave oven, and heated at a power of 510W for 30min to complete the esterification reaction until no more When the water droplets are generated. After the reacted mixture was dried and cooled in a vacuum oven, 40ml of anhydrous dichloromethane was added, shaken thoroughly, filtered, and part of the dichloromethane was evaporated from the filtrate, then 80ml of anhydrous diethyl ether was added to precipitate a white precipitate. The above sample was cooled in a refrigerator at 4-10°C, filtered with suction, and dried to obtain a white solid weighing 1.20 g.
[0028] Add 40ml of dichloromethane to the above crude product to dissolve it, then add 20ml of 10% AcOH / THF to decompose excess dicyclohexylcarbo...
Embodiment 2
[0030] In a 25ml Erlenmeyer flask, add 2.50g (0.5mmol) mPEG-COOH 5000, 0.30g (0.75mmol) 4'-norepipodophyllotoxin, 0.21g (1.0mmol) dicyclohexylcarbodiimide and 0.12 g (1.0mmol) p-N,N-dimethylaminopyridine, fully shaken to mix the reactants evenly, then placed in a Whirlpool microwave oven, and heated at a power of 510W for 30min to complete the esterification reaction until no more When the water droplets are generated. After the reacted mixture was dried and cooled in a vacuum oven, 40ml of anhydrous dichloromethane was added, shaken well, filtered, and part of the dichloromethane was evaporated from the filtrate, then 80ml of anhydrous ether was added to precipitate a white precipitate. The above sample was cooled in a refrigerator at 4-10°C, filtered with suction, and dried to obtain a white solid weighing 2.74 g.
[0031] In the above crude product, add 40ml of dichloromethane to dissolve, add 20ml of 10% AcOH / THF to decompose excess dicyclohexylcarbodiimide, wash with 20m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com