Podophyllinic acid lactone derivant and preparation method thereof
A technology of podophyllotoxin and derivatives, which is applied in the field of preparation of water-soluble podophyllotoxin derivatives, can solve the problems that the preparation method has not been reported, and achieve the benefits of absorption and metabolism, water solubility and fat solubility, and improve dissolution performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
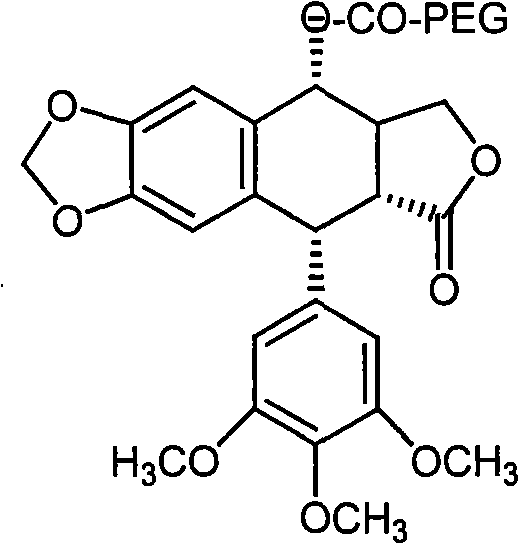
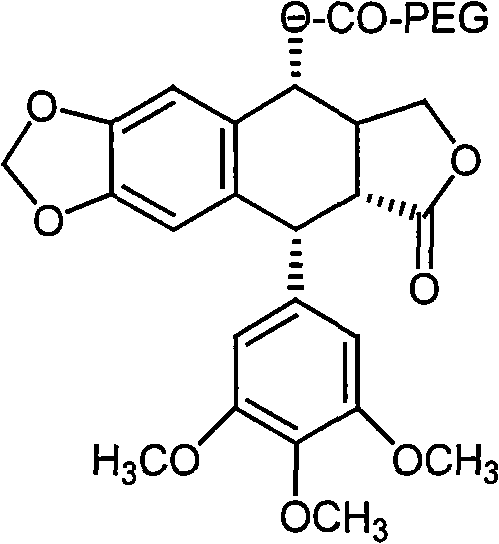
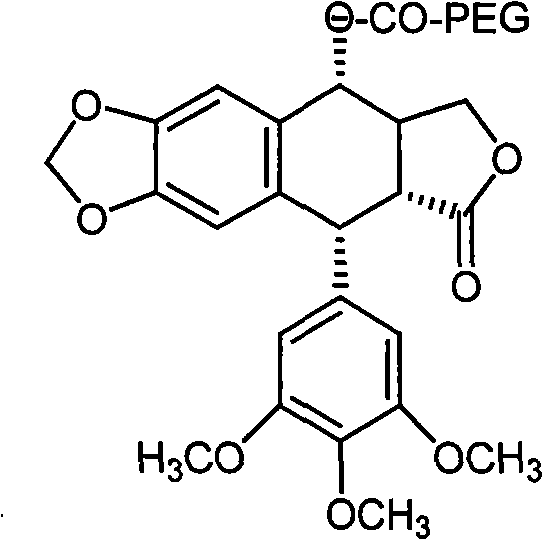
[0022] The preparation route of the present invention is:
[0023] Put podophyllotoxin and carboxy monomethoxypolyethylene glycol in a reaction container, add dicyclohexylcarbodiimide and dimethylaminopyridine to mix the reactants evenly, and react under microwave catalysis for 30 minutes, that is Podophyllotoxin prodrugs can be obtained.
Embodiment 1
[0025] In a 25ml Erlenmeyer flask, add 1.00g (0.5mmol) mPEG-COOH 2000, 0.31g (0.75mmol) podophyllotoxin, 0.21g (1.0mmol) dicyclohexylcarbodiimide and 0.12g (1.0mmol) -N,N-Dimethylaminopyridine, shake well to mix the reactants evenly, then place in a Whirlpool microwave oven, and heat for 30min at a power of 510W until no more water droplets are formed. After the reacted mixture was dried and cooled in a vacuum oven, 40ml of anhydrous dichloromethane was added, shaken thoroughly, filtered, and part of the dichloromethane was evaporated from the filtrate, then 80ml of anhydrous diethyl ether was added to precipitate a white precipitate. The above sample was cooled in a refrigerator at 4-10°C, filtered with suction, and dried to obtain a white solid weighing 1.22 g.
[0026] Add 40ml of dichloromethane to the above crude product to dissolve it, then add 20ml of 10% AcOH / THF to decompose excess dicyclohexylcarbodiimide, wash with 20ml of 0.1N hydrochloric acid solution after 10min...
Embodiment 2
[0028] In a 25ml Erlenmeyer flask, add 2.50g (0.5mmol) mPEG-COOH 5000, 0.31g (0.75mmol) podophyllotoxin, 0.21g (1.0mmol) dicyclohexylcarbodiimide and 0.12g (1.0mmol) -N,N-Dimethylaminopyridine, shake well to mix the reactants evenly, then place in a Whirlpool microwave oven, and heat for 30min at a power of 510W until no more water droplets are formed. After the reacted mixture was dried and cooled in a vacuum oven, 40ml of anhydrous dichloromethane was added, shaken thoroughly, filtered, and part of the dichloromethane was evaporated from the filtrate, then 80ml of anhydrous diethyl ether was added to precipitate a white precipitate. The above sample was cooled in a refrigerator at 4-10°C, filtered with suction, and dried to obtain a white solid weighing 2.66 g.
[0029] In the above crude product, add 40ml of dichloromethane to dissolve, add 20ml of 10% AcOH / THF to decompose excess dicyclohexylcarbodiimide, wash with 20ml of 0.1N hydrochloric acid solution after 10min, add a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com