Preparation method for total synthesis of 4'-demethylepipodophyllotoxin
A demethylated epipodophyllin and total synthesis technology, applied in the direction of asymmetric synthesis, organic chemical methods, chemical instruments and methods, etc., can solve the problems of complex reaction operations and difficult purification of crude products, and achieve environmental friendliness and save steps And the effect of low processing and production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of compound 2:
[0043] Dissolve 18.1 g of compound 1 syringaldehyde in a three-necked reaction flask filled with 180 mL of tetrahydrofuran, control the temperature at 25-30° C., and stir. Add 19.5g of benzyl chloroformate into the reaction flask in the previous step, stir and drop 115mL of 1N sodium hydroxide solution, the dropping time is controlled at 1.5-2 hours, the temperature is controlled at 25-30°C, after the dropping is completed, stir for 30 minute. After the reaction was completed, the liquids were separated, the organic phase was washed with salt water until neutral, the organic phase was concentrated to dryness, and dried under vacuum at 45° C. by adding phosphorus pentoxide for 8 hours to obtain the product compound 2: 30.9 g, Y=98%.
[0044] Synthesis of compound 4:
[0045] Add 12.1g of compound 3 into a three-necked reaction flask containing 210mL of tetrahydrofuran, under nitrogen protection, freeze the reaction solution to -20~-15°C, slow...
Embodiment 2
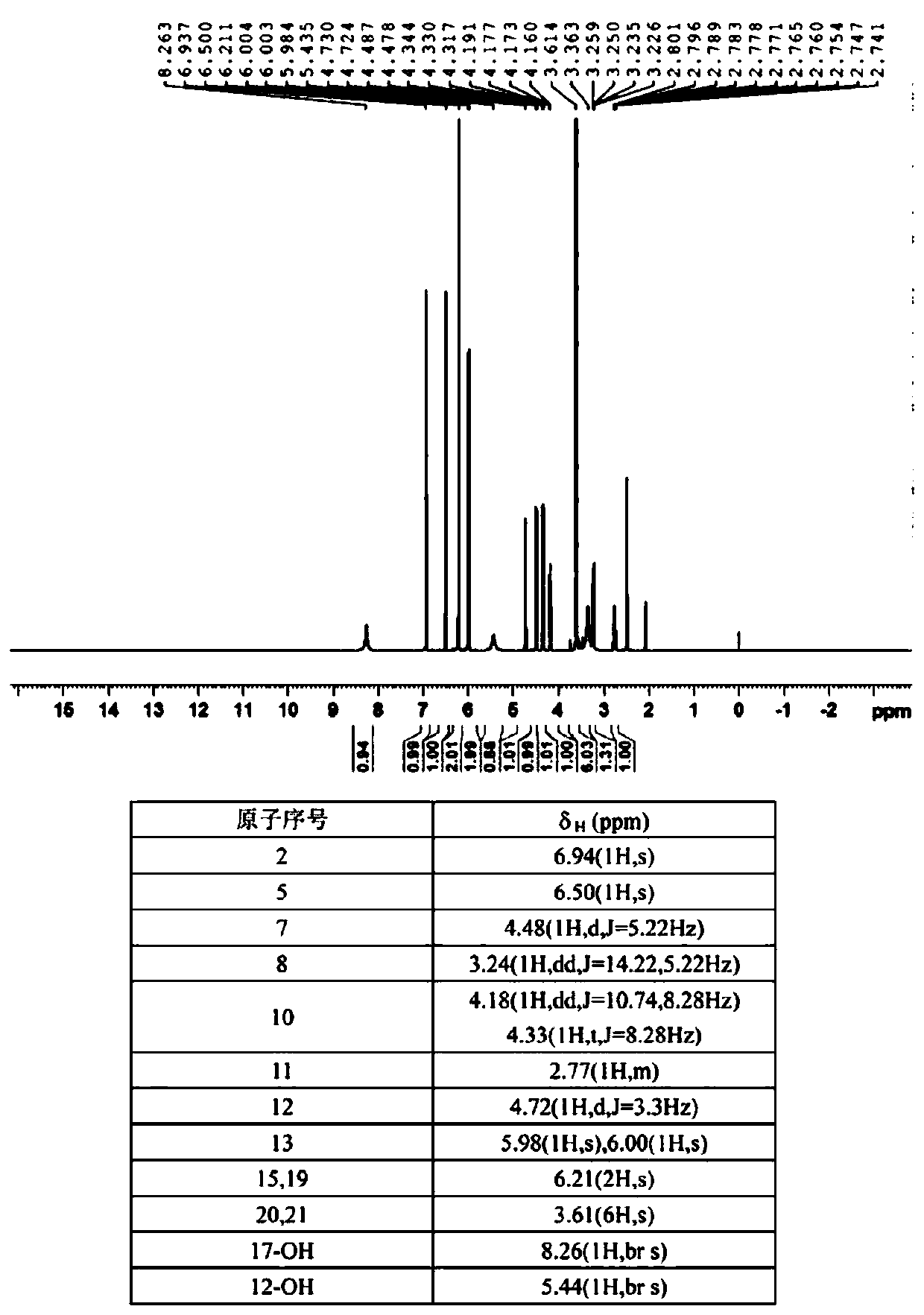
[0057] figure 1 HNMR and its analysis of the product prepared for the present invention.
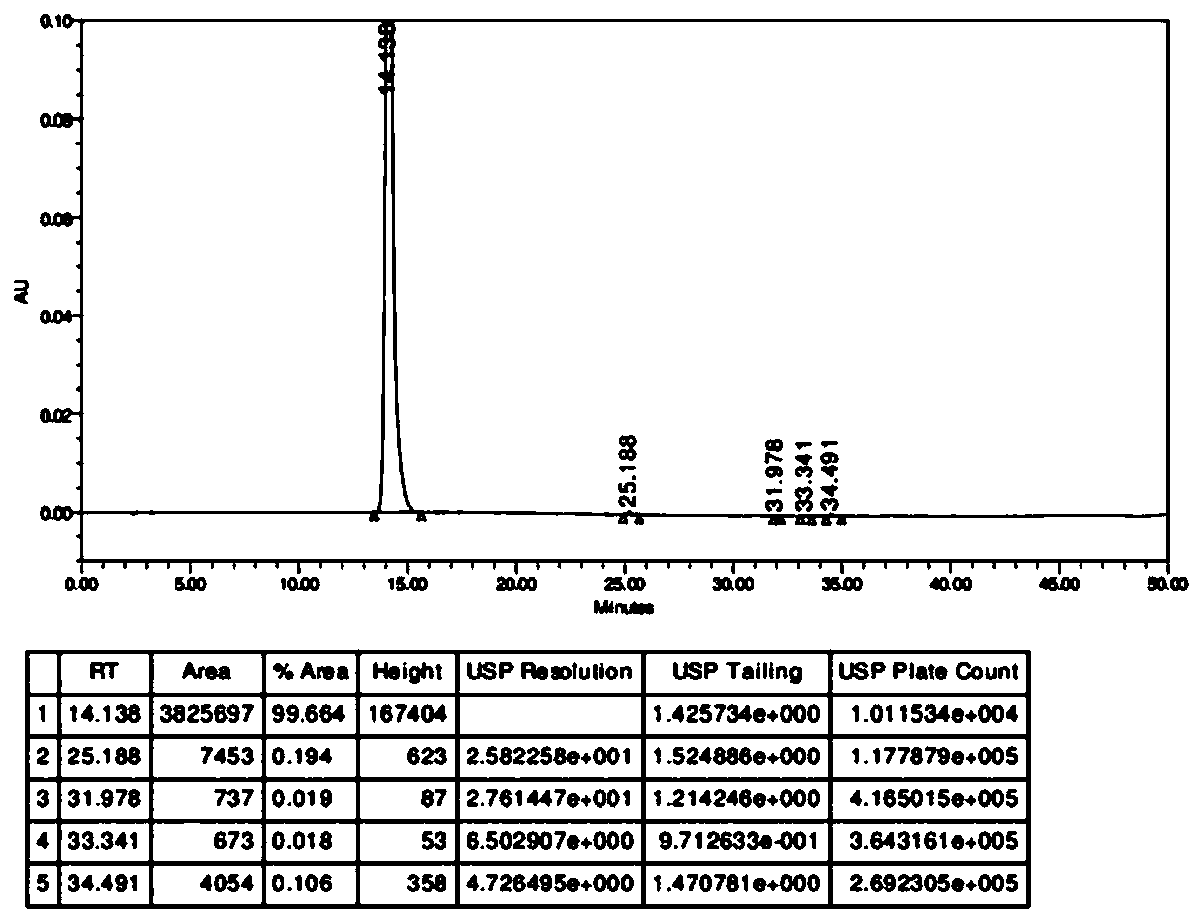
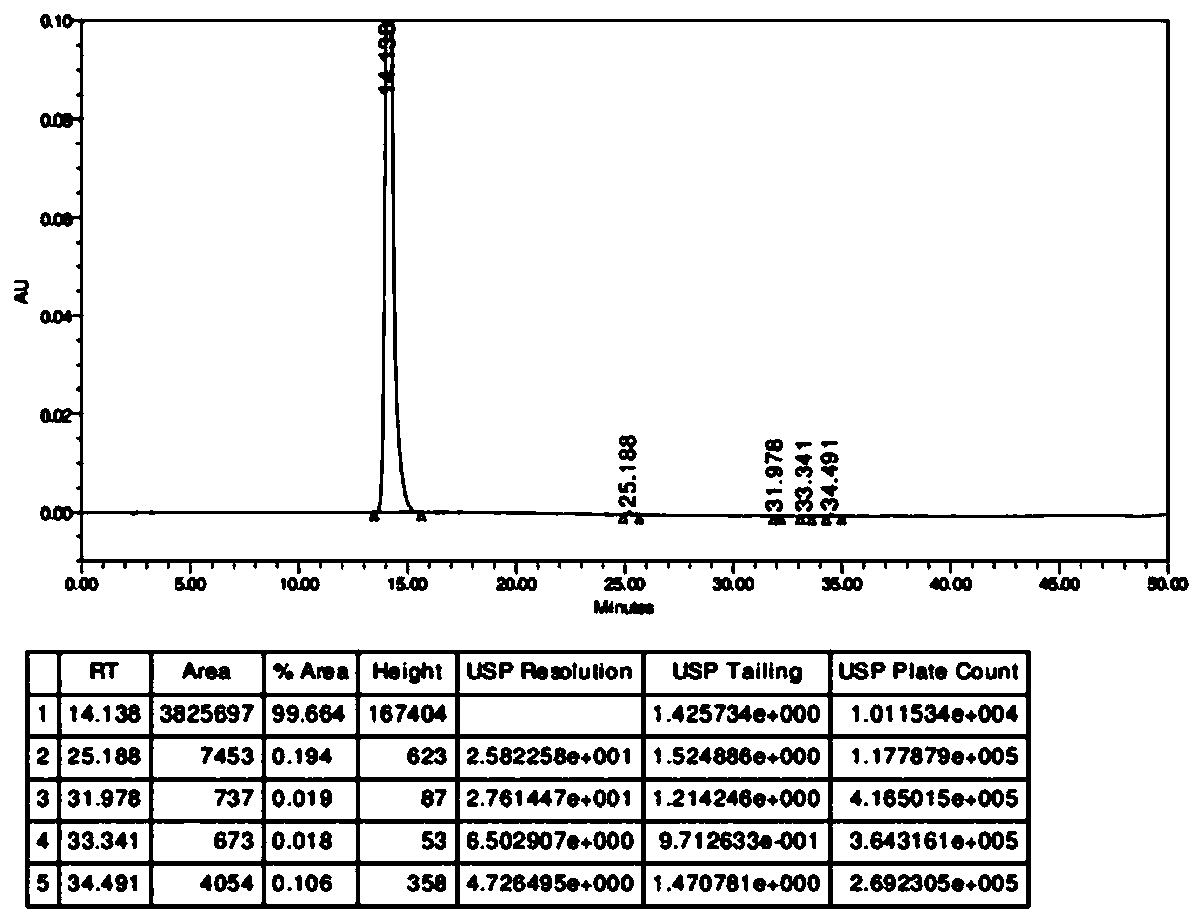
[0058] figure 2 Obtain high performance liquid phase chromatograms for the product prepared by the present invention.
[0059] Adopt conventional high performance liquid phase to detect the product prepared by the present invention, such as figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com