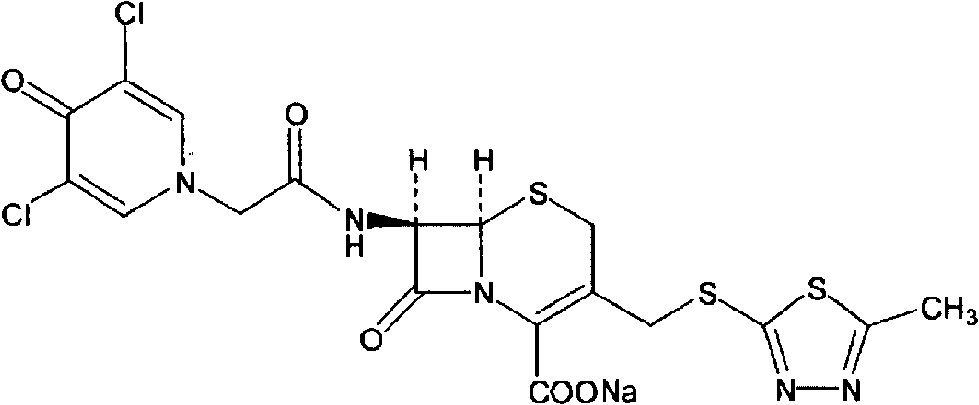
Cefazedone sodium medicament powder injection and method for synthesizing raw medicine of Cefazedone sodium
A technology of cefoxidone sodium and fooxidone sodium powder, which is applied in the directions of pharmaceutical formulations, antibacterial drugs, powder delivery, etc., can solve the problems of unsuitability for industrialized large-scale production, low cefoxidone yield, shortened synthesis route, etc. problem, to achieve the effect of easy control, simplified purification process and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
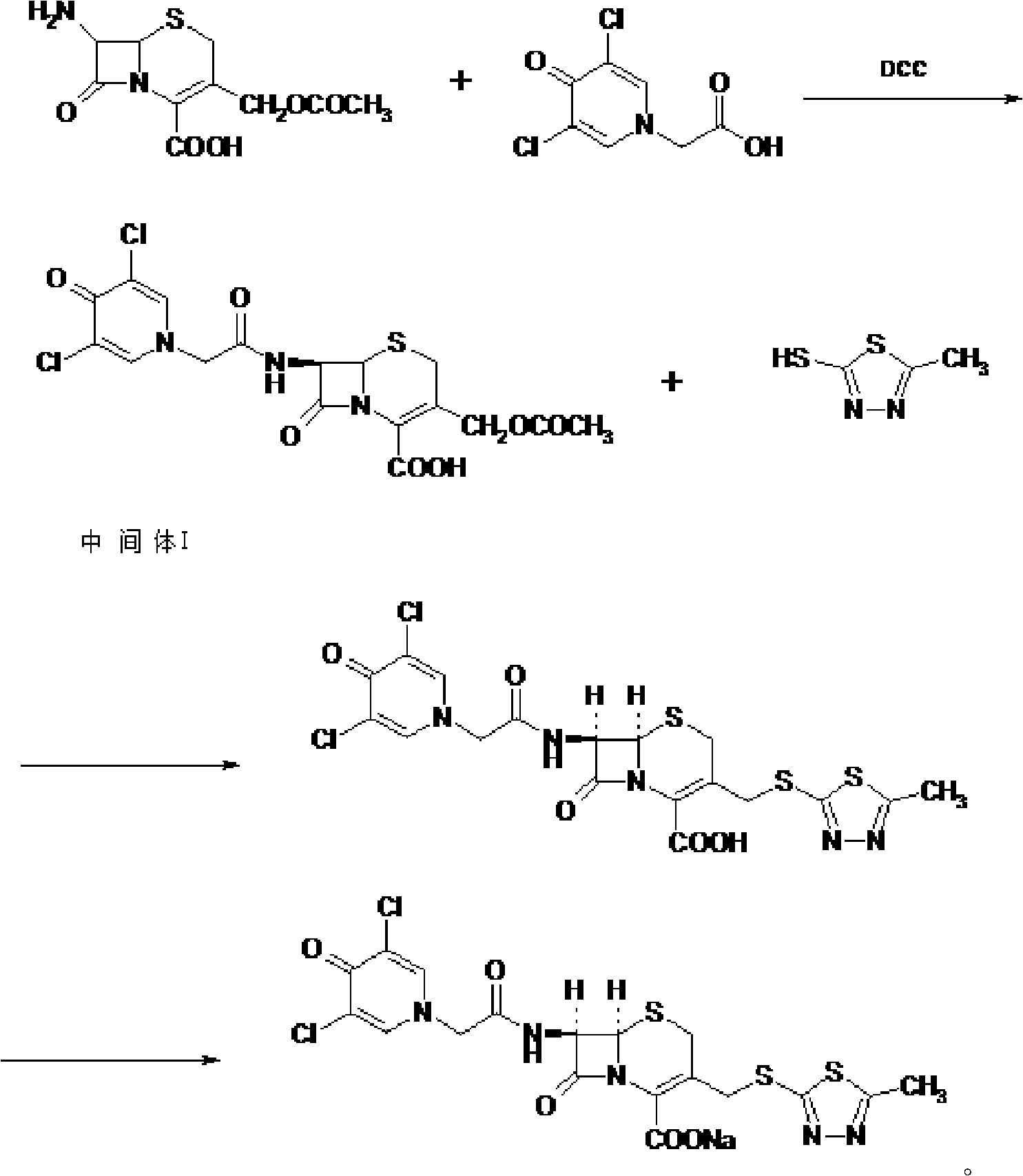
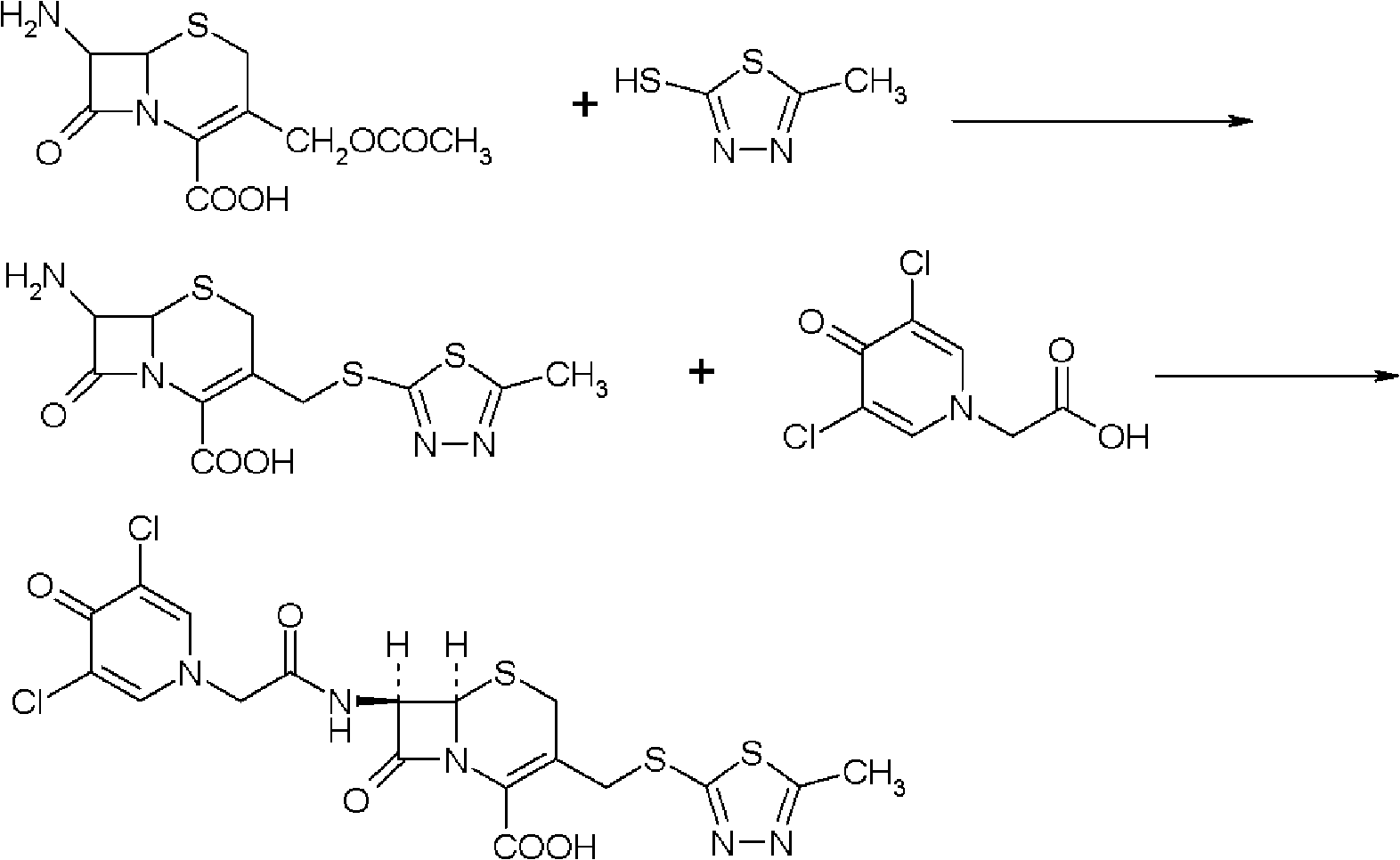
[0054] Synthesis of Intermediate I:
[0055] Put 20g (0.0735mol) of 7-ACA into the flask, add 200ml of tetrahydrofuran, 7.5g of triethylamine, add 17.2g (0.085mol) of dicyclohexylcarbodiimide (DCC), and add dichloromethane / DMF= 1 / 1 (v / v) solution 200ml, cool down to 0°C in an ice bath, add 17.6g (0.0793mol) of 3,5-dichloropyridoneacetic acid, and add 3,5-dichloropyridoneacetic acid per minute After adding 1 / 20 of the total amount, the reaction was stirred at 20°C for 0.5 hour. After filtration, the filtrate was evaporated to dryness under reduced pressure at 50°C, recrystallized from diethyl ether, refrigerated for crystallization for more than 8 hours, filtered, and vacuum-dried at 50°C to obtain 33.1 g of intermediate I, with a yield of 94.5%. The infrared characteristic absorption peak that obtains intermediate I is: 3400cm -1 , 1654.48cm -1 , 1600cm -1 , 1400cm -1 .
Embodiment 2
[0057] Synthesis of Intermediate I:
[0058] Put 20g (0.0735mol) of 7-ACA into the flask, add 200ml of dichloromethane, 7.5g of triethylamine, add 19.68g (0.0955mol) of dicyclohexylcarbodiimide (DCC), and add dichloromethane solution 200ml, cool down to -5°C in an ice bath, add 19.6g (0.0882mol) of 3,5-dichloropyridoneacetic acid, and add 1 / 10 of the total amount of 3,5-dichloropyridoneacetic acid per minute. , and stirred at 25°C for 0.5 hours. After filtration, the filtrate was evaporated to dryness under reduced pressure at 50°C, recrystallized from diethyl ether, refrigerated for crystallization for more than 8 hours, filtered, and vacuum-dried at 50°C to obtain 33.7g of intermediate I, with a yield of 96.4%. The infrared characteristic absorption peak that obtains intermediate I is: 3400cm -1 , 1654.48cm -1 , 1600cm -1 , 1400cm -1 .
Embodiment 3
[0060] Synthesis of Intermediate I:
[0061] Put 20g (0.0735mol) of 7-ACA into the flask, add 200ml of N,N-dimethylformamide, 7.5g of triethylamine, and 15.2g (0.0735mol) of dicyclohexylcarbodiimide (DCC), Add 200ml of dichloromethane / DMF=1 / 1 (v / v) solution, cool down to 0°C in an ice bath, add 24.47g (0.11mol) of 3,5-dichloropyridoneacetic acid, and add 3 , 1 / 15 of the total amount of 5-dichloropyridoneacetic acid was added, and the reaction was stirred at 35° C. for 0.5 hours. After filtration, the filtrate was evaporated to dryness under reduced pressure at 50°C, recrystallized from diethyl ether, refrigerated for crystallization for more than 8 hours, filtered, and vacuum-dried at 50°C to obtain 31.7g of intermediate I, with a yield of 90.6%. The infrared characteristic absorption peak that obtains intermediate I is: 3400cm -1 , 1654.48cm -1 , 1600cm -1 , 1400cm -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com