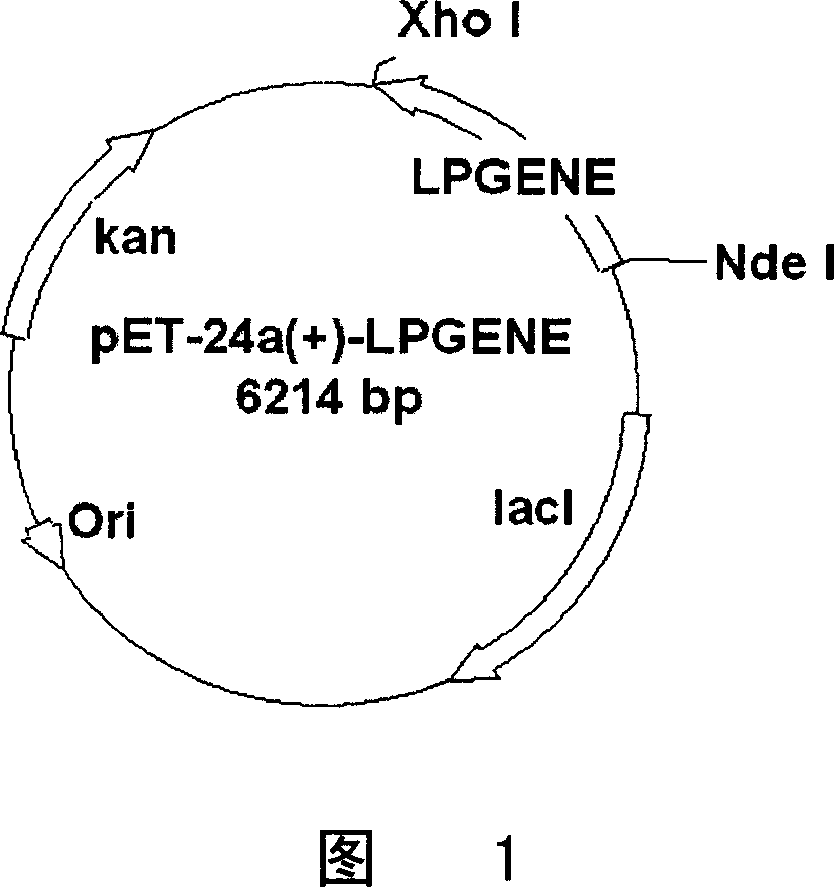
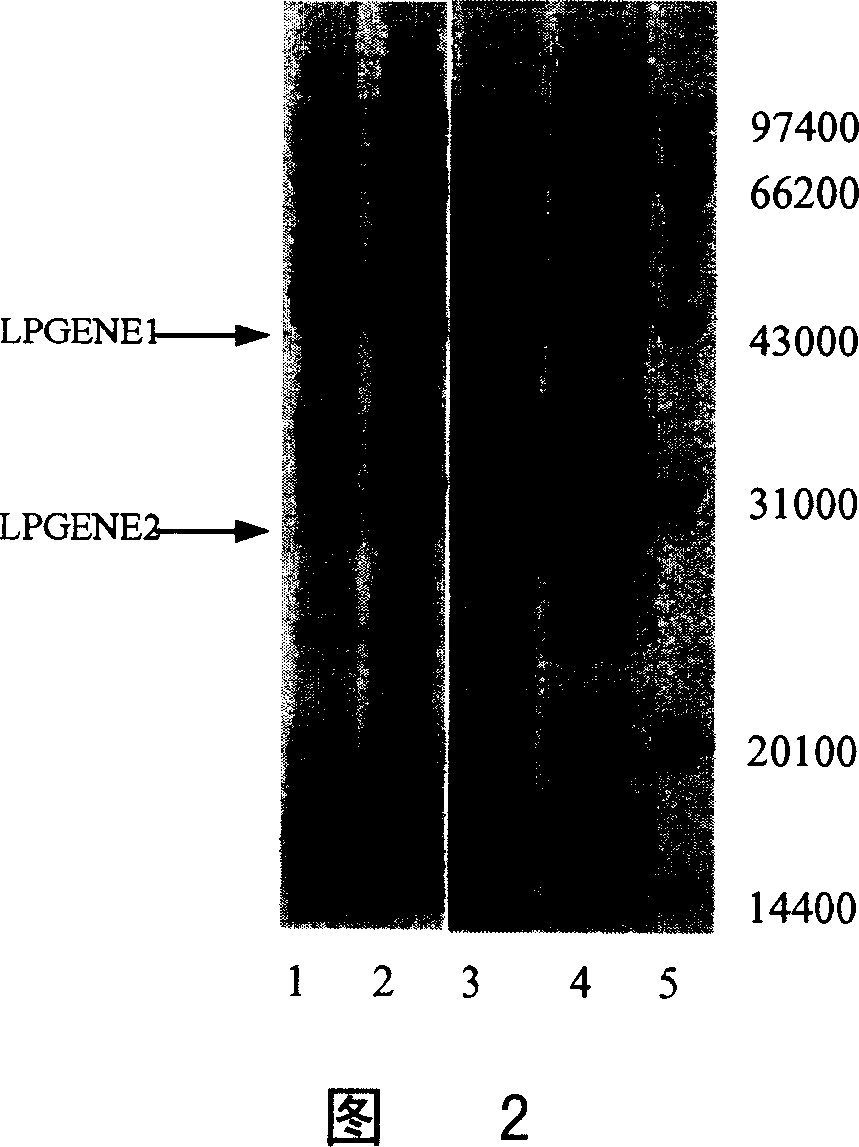
Preparation and purification of telomerase activity inhibition protein
An activity inhibitor, a technology of telomerase, which is applied in the fields of biotechnology and biochemical engineering, and can solve the problems of high production cost and inappropriate mass preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Preparation and induced expression of genetically engineered bacteria of embodiment 1.LPGENE1 and LPGENE2
[0081] (a) Preparation of LPGENE1 and LPGENE2 genes
[0082] Using the full-length LPTS gene sequence (see Chinese patent ZL00115395.1 for the preparation method) as a template, use the following primers,
[0083] P1 (upstream primer): 5'-CATATGTCTATGCTGGCTGAACGTCGG-3' (SEQ ID NO: 7)
[0084] P2 (downstream primer): 5'-CTCGAGTTTGGAATCTTTCTTCTT-3' (SEQ ID NO: 8)
[0085] Through the PCR reaction, obtain the PCR amplification product (LPGENE1 gene) containing the full-length LPTS coding sequence with restriction sites NdeI and Xho1 at both ends, the sequence is shown in SEQ ID NO: 1; PCR reaction conditions are: in 50 μ l system To proceed, include 1 μl template, 1 μl dNTP mix (10 mM), 1 μl each of upstream and downstream primers (20 μM), 5 μl 10×PYROBEST buffer, 0.5 μl PYROBEST enzyme, and add deionized water to 50 μl. The reaction conditions were: 94°C for 3 mi...
Embodiment 2
[0100] Example 2. Separation and purification of LPGENE1 and LPGENE2
[0101] A small amount of purified LPGENE protein prepared in the laboratory can be used for affinity chromatography with a commercially available Hi-Sepharose 4B column. In order to prepare a large amount of purified LPGENE protein, separation methods such as ion exchange were used in this example to obtain high-purity LPGENE protein.
[0102] The specific separation and purification process may include the following steps:
[0103] (1) Ultrasonic disruption of bacteria
[0104] Get the thalli of centrifugation gained, add the buffer solution (30mmol / L KH 2 PO 4 -NaOH) to resuspend the cells, and then add 2‰ of 100mM PMSF (phenylmethylsulfonyl fluoride) (final concentration 0.2mM). Then under the condition of ice bath, the cell is ultrasonically broken, (ultrasonic instrument, manufactured by Ningbo Xinzhi Company), the ultrasonic conditions are as follows: working time 7 seconds, interval time 25 secon...
Embodiment 3
[0117] Example 3. Detection of biological activity of LPGENE1 and LPGENE2
[0118] LPGENE is a strong inhibitor of telomerase activity. The present invention uses the TPAP method to measure the biological activity of the LPGEGE prepared and purified in Example 2.
[0119] Telomerase comes from the lysate of conventional SMMC-7721 liver cancer cells. The specific preparation method is as follows: Inoculate SMMC-7721 liver cancer cells in a 10ml culture flask, the medium is RPMI 1640, the temperature is 37 ° C, 5% CO 2 Under the condition of adherent culture, collect the cultured cells after the bottle is full. The culture medium was aspirated dry, the cells were washed once with PBS, and then the cells were digested with trypsin, washed with PBS, sucked into a centrifuge tube, and centrifuged at 4000rpm to collect the cells. Then wash buffer (10mM Hepes-KOH (pH7.5), 1.5mM MgCl 2, 10mM KCl, 1mMDTT (dithiothreitol)) wash the cells once, and centrifuge. Use ice-cold lysis buff...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com