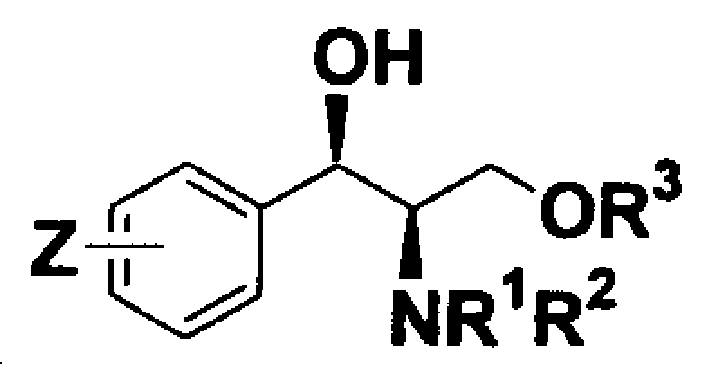
Chiral alkamine ligand and its application in asymmetrical addition of terminal alkyne para imine
A technology of chiral amino alcohols and ligands, applied in asymmetric synthesis, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., can solve problems such as difficult industrialization and harsh conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of (1R, 2R)-2-N,N-dimethylamino-3-p-nitrophenyl-1,3-propanediol:
[0031] Reference Jiang, B.; Chen, Z.L.; Tang, X.X. Org. Lett. 2002, 4, 3451. Synthesis
Embodiment 2
[0033] Preparation of (1R, 2R)-3-tert-butoxy-2-N,N dimethylamino-1-p-nitrophenyl-1-propanol:
[0034] At 0-5°C, 0.8 g of concentrated sulfuric acid was added dropwise to (1R, 2R)-2-N,N-dimethylamino-3-p-nitrophenyl-1,3-propanediol (1.8g, 7.5mmol) in CH 2 Cl 2 (20mL) in solution. Keep isobutylene gas at 0-5°C for one hour. Then 0.2 g of concentrated sulfuric acid was added dropwise, the mixture was returned to room temperature and stirred vigorously for 5-7 hours, and isobutylene gas was continuously introduced. Cool the mixture to 0-5℃ and add saturated K 2 CO 3 Solution. Organic phase drying (Na 2 SO 4 ) Concentrated and recrystallized to obtain the ligand 1.44g (65%).mp 100.0-101.3℃; [α] D 20 = +23.5(c, 1.00, CHCl 3 ); FTIR (KBr) 3333, 2972, 1606, 1523, 1357, 1197, 861cm -1 ; 1 HNMR(300MHz, CDCl 3 )δ8.19(d, J=8.8Hz, 2H), 7.60(d, J=8.4Hz, 2H), 4.59(d, J=9.9Hz, 1H), 3.34(dd, J=3.0Hz, and 9.9 Hz, 1H), 3.21 (dd, J=6.5 Hz, and 10 Hz, 1H), 2.56 (m, 1H), 2.47 (s, 6H), 1.06 (s, 9H); 13...
Embodiment 3
[0035] Example 3 Preparation of (1R, 2R)-3-tert-butyldimethylsiloxy-2-N,N dimethylamino-1-p-nitrophenyl-1-propanol: (1R, 2R)- 2-N,N-Dimethylamino-3-p-nitrophenyl-1,3-propanediol (1.946g, 8.1mmol) dissolved in CH 2 Cl 2 (30mL), add TBDMSCl (1.28g, 5.3mmol) and imidazole (1.4g, 20.6mmol) mixture at 0°C and stir overnight. After processing, the product is 2.72g. FTIR (KBr) 3344, 2954, 1606, 1525, 1349cm -1 ; 1 HNMR(300MHz, CDCl 3 )δ8.25-8.20(d, J=8.5Hz, 2H), 7.6-7.55(d, J=8.5Hz, 2H), 4.65(d, J=9.7Hz, 1H), 3.77-3.6(dd, J =11.3Hz, 2.7Hz 1H), 3.5-3.45 (dd, J=11.3Hz, 6.0Hz 1H 2.50(m, 7H), 1.85(s, 8H), 0.1(s, 6H); 13 CNMR(75MHz, CDCl 3 )δ150.2, 147.4, 128.0, 123.3, 69.0, 57.1, 41.6, 25.7, 17.9, -5.9; MS (EI) m / e297 (M+-57, 0.3), 209 (8.2), 202 (100). Anal .calcd.for C 17 H 30 N 2 O4 Si: C, 57.60; H, 8.53; N, 7.90. Found: C, 57.82; H, 8.18; N, 7.77.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com