Bisbenzylisoquinoline compounds, preparation method and applications
A technology of bisbenzylisoquinoline and compounds, applied in the field of medicine, can solve problems such as no reports of anti-tumor cytotoxic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
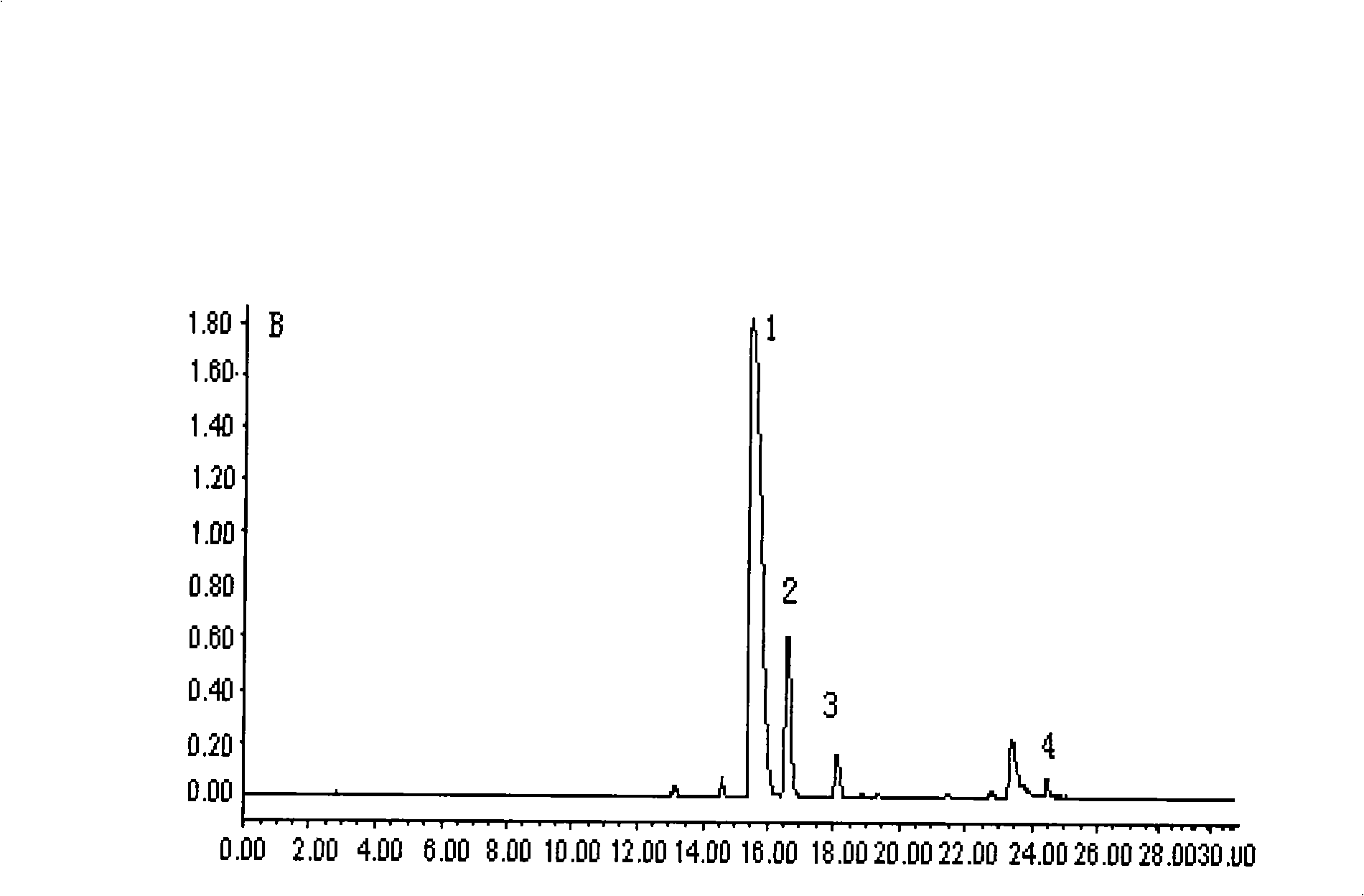
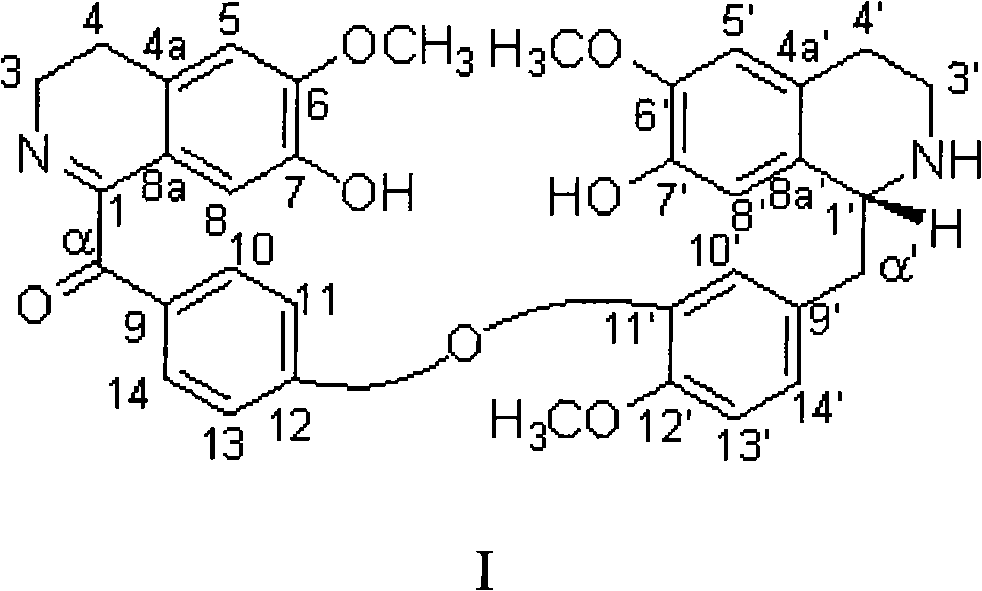
[0014] The physicochemical and UV, IR, MS spectral data of embodiment 1 compound I are as follows:
[0015] Compound I is a pale yellow solid, [α] D 20 -16°(c 1.0, MeOH); electrospray mass spectrometry (ESIMS) (positive ion type positive) m / z 595[M+H] + (100); (negative ion negative) m / z 593(100); UV(MeOH)λ max nm(logε): 207(5.30), 288(4.87); IR(KBr)v max cm -1 3405, 2937, 2842, 1664, 1588, 1510, 1450, 1371, 1276, 1228, 1135, 1028, 756.
[0016] Compound I 1 H NMR, 13 C NMR data and their assignments are shown in Table 1. The assignments of each hydrogen and carbon signal were obtained by testing two-dimensional NMR spectra (carbon-hydrogen correlation spectrum, carbon-hydrogen long-range correlation spectrum, nuclear Overhauser effect two-dimensional correlation spectrum).
[0017] Table 1. Compound I 1 H(400MHz) and 13 C (100MHz) NMR data and signal attribution (CD 3 OD solvent)
[0018] no
[0019] 14′
Embodiment 2
[0021] Cut 5kg of dried root tubers of black medicine into thin slices, add 50L ethanol after crushing, and extract twice by percolation. After combining the two extracts, concentrate under reduced pressure to obtain the extract, suspend and dissolve the extract with 10L of water, and dissolve the extract with 2N HNO 3 After acidification, it was extracted three times with an equal volume of ethyl acetate, and the ethyl acetate layer was discarded after recovering the solvent. 5% Na for the aqueous phase 2 CO 3 Adjust the pH to 10, extract twice with an equal volume of ethyl acetate, combine the ethyl acetate extracts, wash with distilled water until neutral, and recover ethyl acetate under reduced pressure to obtain 32.3 g of crude total alkaloids. Dissolve the crude product in 3L of ethanol, add water to make the ethanol content 60%, let it stand for complete precipitation, filter and dry to obtain 12.8g of refined total alkaloids.
[0022] Take the above-mentioned refine...
Embodiment 3
[0024] Cut 3kg of dried root tubers of black medicine into thin slices, add 30L methanol after crushing, and reflux extraction twice, the first time is 2 hours, after the extract is filtered, add 24L methanol to continue reflux extraction for 1 hour. The two extracts were combined and then concentrated under reduced pressure to obtain an extract, which was suspended and dissolved in 6 L of water, acidified with 3N HCl, extracted three times with an equal volume of chloroform, and the chloroform layer was discarded after recovering the solvent. The pH of the aqueous phase was adjusted to 10 with ammonia water, extracted twice with an equal volume of chloroform, the combined chloroform extracts were washed with distilled water until neutral, and 21.7 g of crude total alkaloids were obtained after recovering the chloroform under reduced pressure. Dissolve the crude product in 3L of methanol, add water to make the methanol content 50%, let it stand for complete precipitation, filte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com