Tyrosinase polypeptide inhibitor
A technology of tyrosinase and inhibitors, which is applied in the field of tyrosinase inhibitors containing polypeptides, and can solve problems such as immediate effect and safety concerns
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
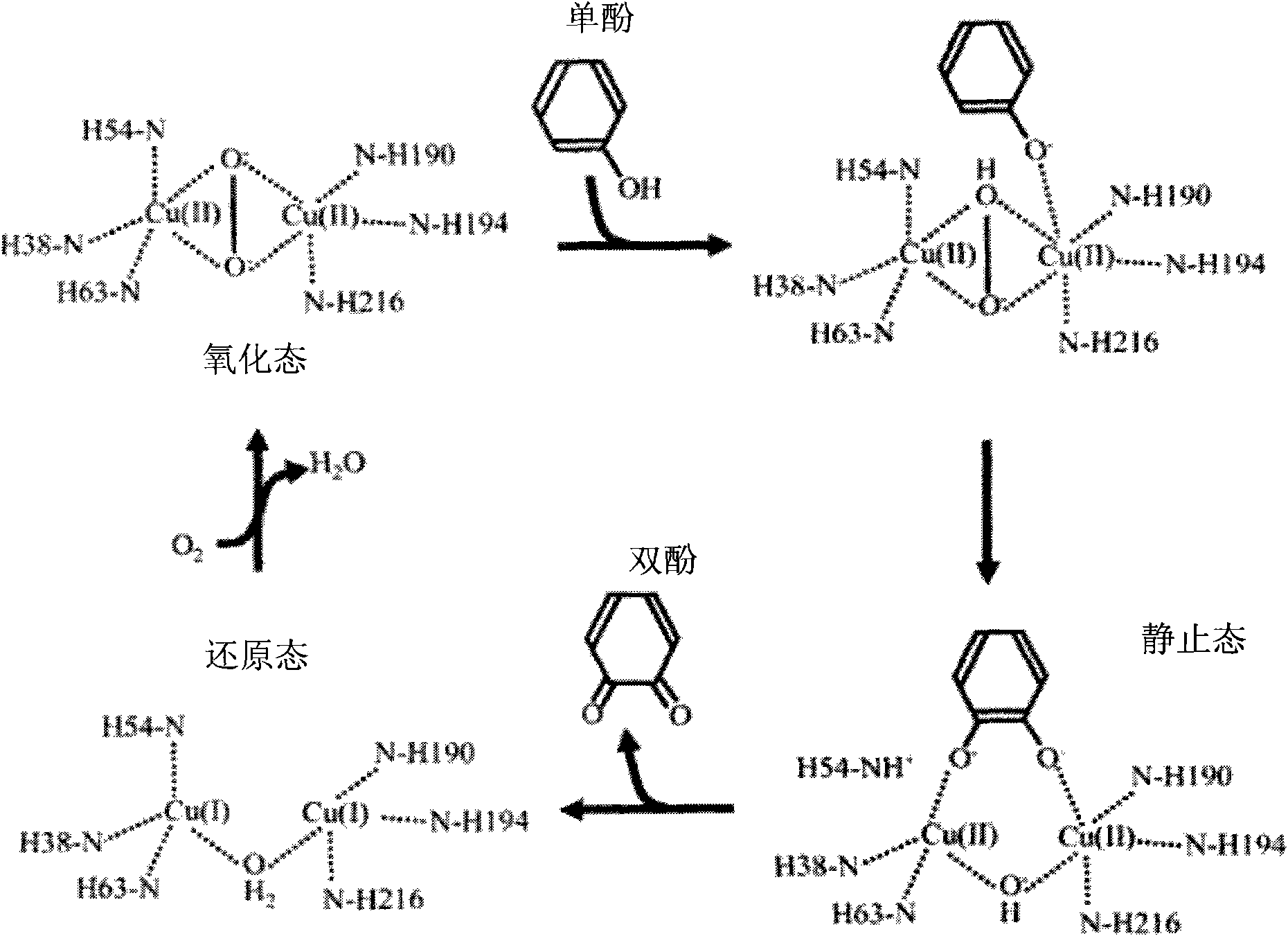
[0119] Embodiment 1: in vitro (In vitro) tyrosinase activity test
[0120] I. Drugs
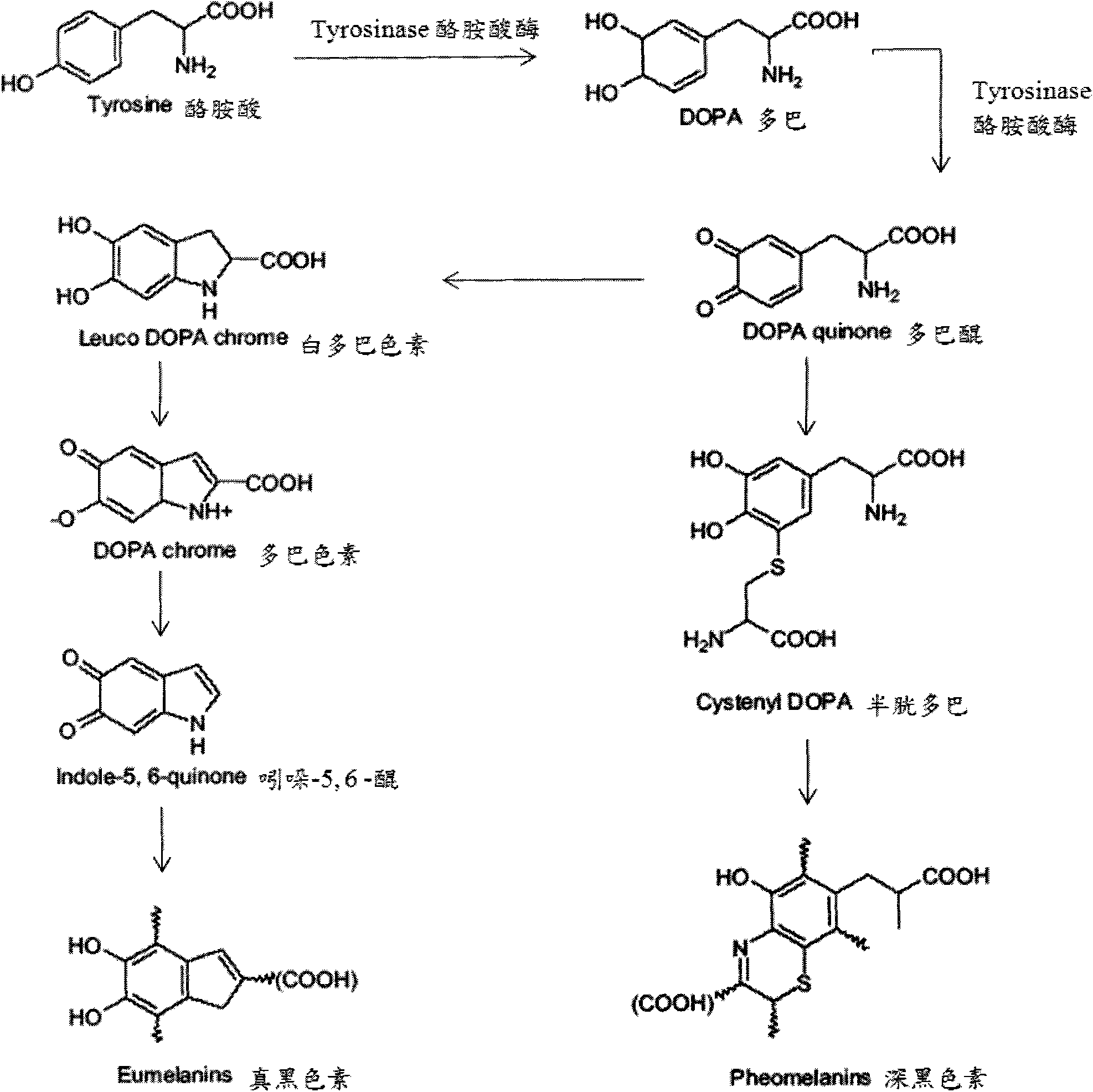
[0121] Mushroom tyrosinase (Mushroom tyrosinase, 100U / ml), arbutin (Arbutin), tyrosine (L-tyrosine, 0.5mM), kojic acid (Kojic acid) and 2 to 5 short polypeptides of different sequences (0.5 mM). The short polypeptide sequence is R1n1-Xaa-R2n2-Yaa-R3n3, Xaa or Yaa is one of the five amino acids of tyrosine, cysteine, glycine, glutamic acid and arginine, and the substituents R1, R2 or The amino acids of R3 are divided into the following eight types according to their functional groups: glycine, aliphatic amino acids, aromatic amino acids, acidic amino acids, basic amino acids, hydroxyl amino acids, sulfur-containing amino acids and amide amino acids, choose one of each type, n1, n2 or n3 The number of substituents is equal to 0 or 1 respectively, and the above amino acids are cross-arranged to form short polypeptides with 2 to 5 different sequences.
[0122] II. Experimental steps
[0123] ...
Embodiment 2
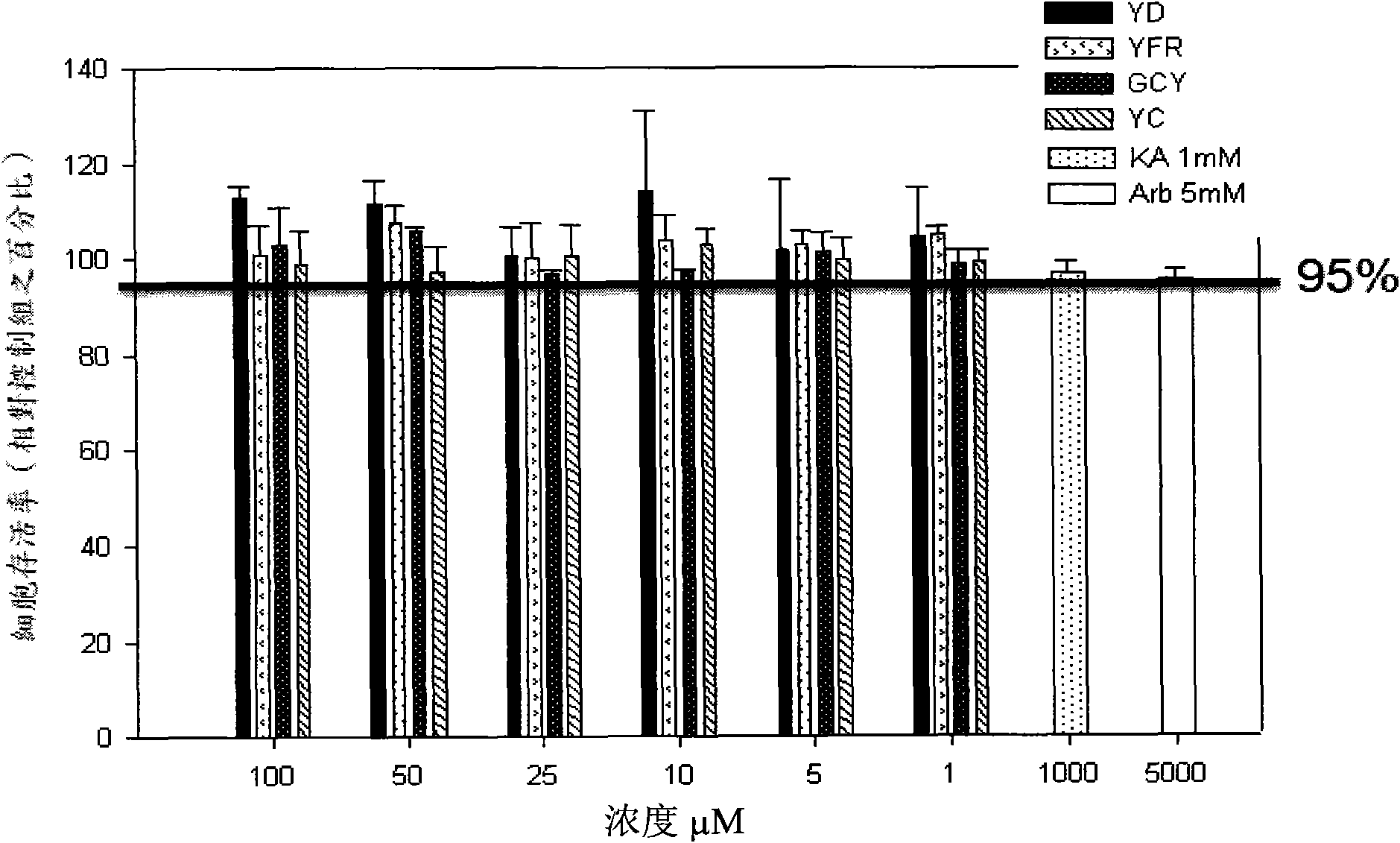
[0149] Example 2: Results of Safety Tests for Short Peptides of Different Sequences
[0150] I. Drug source
[0151] 1. The peptide sequence is YD, YFR, GCY and YC
[0152] II. Experimental steps
[0153] Use the MTT test to determine whether the inhibitor contains cytotoxicity. The succinate dehydrogenase (succinate dehydrogenase) in the mitochondria of living cells can reduce the yellow MTT to purple crystals (formazan), and then add DMSO to the purple crystals. (formazan) was dissolved, and the absorbance value of this solution at OD 595nm was measured with a spectrophotometer, and the effect of the test substance on cell growth was estimated.
[0154] (1) Prepare MTT-containing culture medium first, and add 80 μl of MTT (original concentration: 5 mg / ml) to every 500 μl of culture medium.
[0155] (2) Aspirate all the cell culture medium, add 100 μl of MTT-containing medium to each 96-well plate (500 μl of MTT-containing medium in a 24-well plate), and incubate at 37° C....
Embodiment 3
[0159] Example 3: Analysis of melanin content in vivo (In vivo)
[0160] In this patent, there are some cis-reverse compounds whose inhibitory effect will not be different due to cis-reverse, but there are also some cis-reverse compounds whose inhibitory effects can differ by about 50%. On the other hand, Some compounds have color after being dissolved in the solution, so in order to eliminate the color may affect the high absorbance value, and confirm that the inhibitor designed in this study can also have the same good whitening effect in the human body, so we choose Some compounds YD, YFR, GCY, YC were analyzed for melanin content.
[0161] I. Drug source
[0162] 1. The peptide sequence is YD, YFR, GCY and YC
[0163] II. Experimental steps
[0164] Melanocytes were cultured in 24-well plates, and different inhibitors were cultured at concentrations of 1, 5, 10, 25, 50 and 100 μM for seven days. Seven days later, trypsin / EDTA (0.25% / 0.1% inphosphate bufferedsaline) was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com