Application of liposome to treatment of chronic viral hepatitis B
A technology of viral hepatitis and liposome, which is applied in the direction of liposome delivery, antiviral agents, organic active ingredients, etc., can solve the problems of chronic hepatitis B virus without liposome, and achieve good stability in vitro , The preparation process is simple, and the effect of maintaining stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
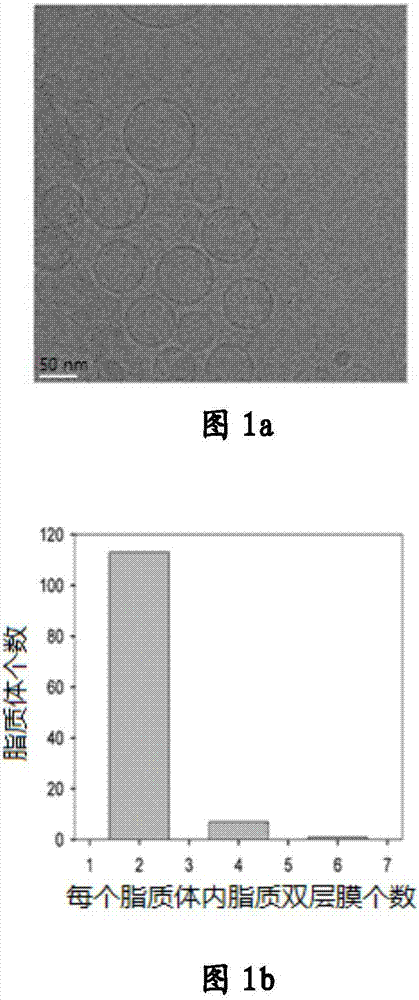
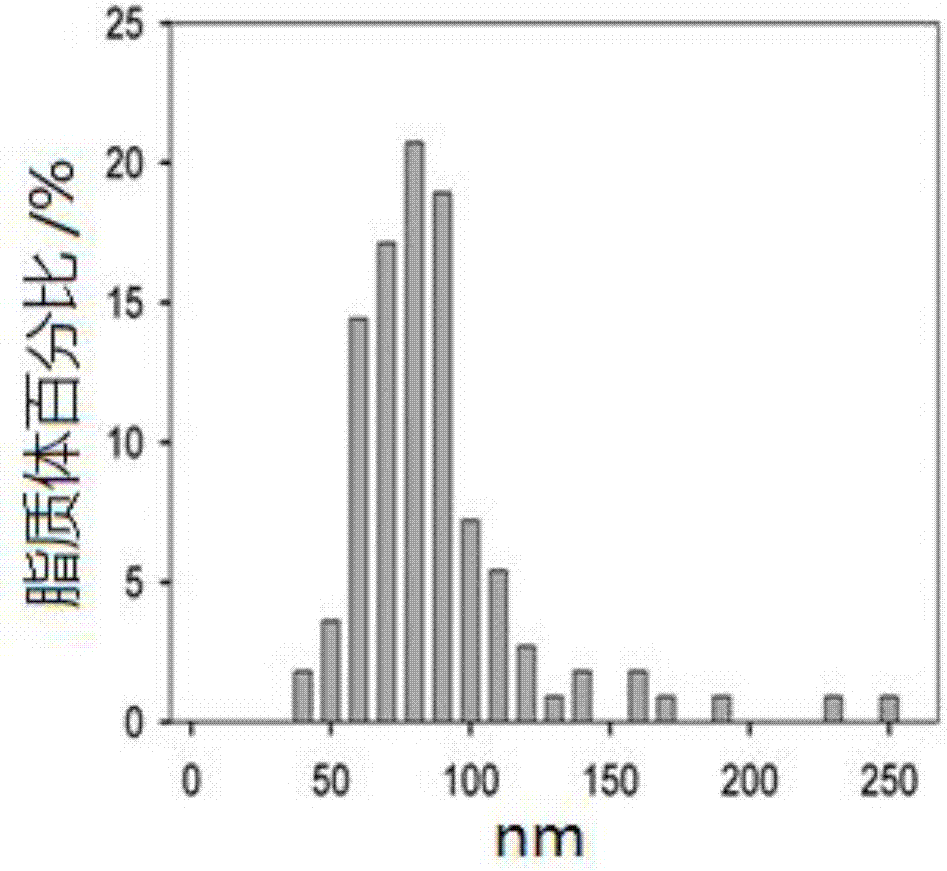
[0029] Embodiment 1: liposome preparation and property analysis
[0030] (1) Liposome preparation, concentration and lyophilization:
[0031] The following raw materials were used to prepare the liposomes of this embodiment: soybean lecithin, cholesterol, palmitic acid, vitamin E, mannitol (20% sterile aqueous solution), human albumin (concentration is 20% solution), ether, ethanol and phosphate buffer (pH 6.5, 0.1 mM).
[0032]The liposomes were prepared by high-pressure injection and secondary emulsification. Add 14.1166g of soybean lecithin, 2.3202g of cholesterol, 0.4630g of palmitic acid, and 0.8514g of vitamin E to dissolve in 300mL of ether; filter the above solution through a 0.2μm microporous membrane into an emulsification bottle, and then add 300mL of ethanol to form an emulsion ( W / O); inject the obtained above-mentioned emulsion into 14.4L water, control the reaction temperature to be 40°C and stir at the same time, form the emulsion (W / O / W) twice, and gradual...
Embodiment 2
[0038] Embodiment 2: the selection of liposome lyophilization excipient
[0039] Freeze-drying and excipient of liposome: compare the volume ratio of liposome concentrate, different excipients (human albumin, povidone K30) and their concentration on the shrinkage of liposome shape after freeze-drying The results are shown in Table 1.
[0040] Table 1: The volume ratio of liposome concentrate and the effect of excipients and their concentration on lyophilization
[0041]
[0042] Note: *The volume of liposome concentrate accounts for the volume percentage of emulsion.
[0043] As can be seen from Table 1, when liposomes are lyophilized, when the concentrated volume of liposomes is 25-50% of the final emulsion volume, adding 1% human albumin as an excipient can ensure that the lipid The body shape does not shrink after freeze-drying.
Embodiment 3
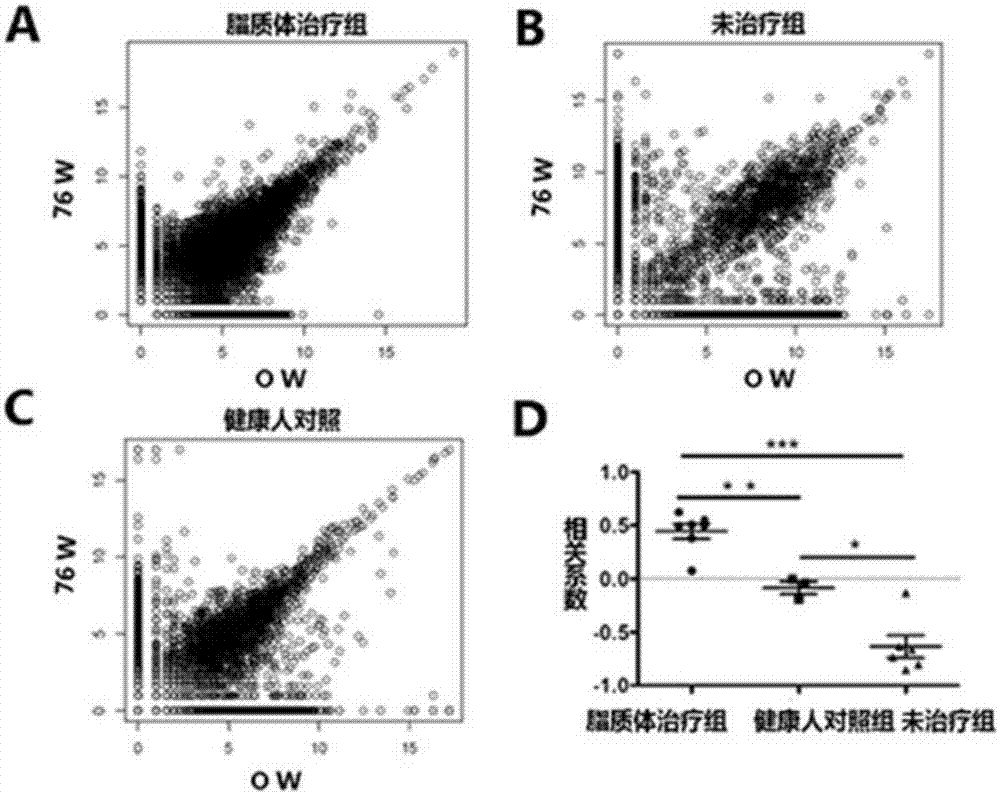
[0044] Embodiment 3: the therapeutic effect of liposome in chronic hepatitis B patient
[0045] In this example, the finished liposome prepared in Example 1 is used to treat selected patients with chronic hepatitis B, and to explore its therapeutic effect and effect on chronic hepatitis B.
[0046] (1) Selection of subjects: 119 patients with chronic hepatitis B were selected as subjects. The details of the subjects are as follows:
[0047]
[0048] (2) Administration method: administer by subcutaneous injection on the upper arm, and give 900 μg liposome finished product each time; 28 weeks subcutaneous injection 6 times.
[0049] (3) Efficacy evaluation: chronic hepatitis B patients were treated with the liposomes of the embodiment of the present invention 1, and the peripheral blood of the test patients was collected respectively at 12, 28, 32, 40, 52, 64, and 76 weeks , HBeAg / anti-HBe conversion rate, serum HBV virus titer and serum alanine aminotransferase (ALT) co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com