125i labeled caerin polypeptide and its application
A labeling and polypeptide solution technology, applied in the biological field, to achieve good in vitro stability, good radiochemical purity, and high labeling rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1125I
[0042] Example 1 125 Preparation of I-labeled F3 polypeptide
[0043] S1. Take lodogen, add chloroform to dissolve it at a solid-to-liquid ratio of 1 mg / mL, take 100 μL and add it to the bottom of the reaction tube, dry it under negative pressure, seal it, and store it at -15°C to -20°C to prepare a reaction coated with lodogen. Tube;
[0044] S2. Take the reaction tube coated with lodogen prepared in step S1, and add 40 μL of F3 polypeptide solution with a concentration of 0.4 μg / μL and 20 μL of Na with a concentration of 25 μCi / μL in sequence. 125 1, stirring reaction 15min under the condition of 25 ℃ at temperature;
[0045] S3. After the reaction time is over, immediately add 300 μL of phosphate buffer solution with a concentration of 0.05 mol / L and a pH of 7.4 to terminate the reaction, and let stand for 5 minutes to obtain a reaction solution;
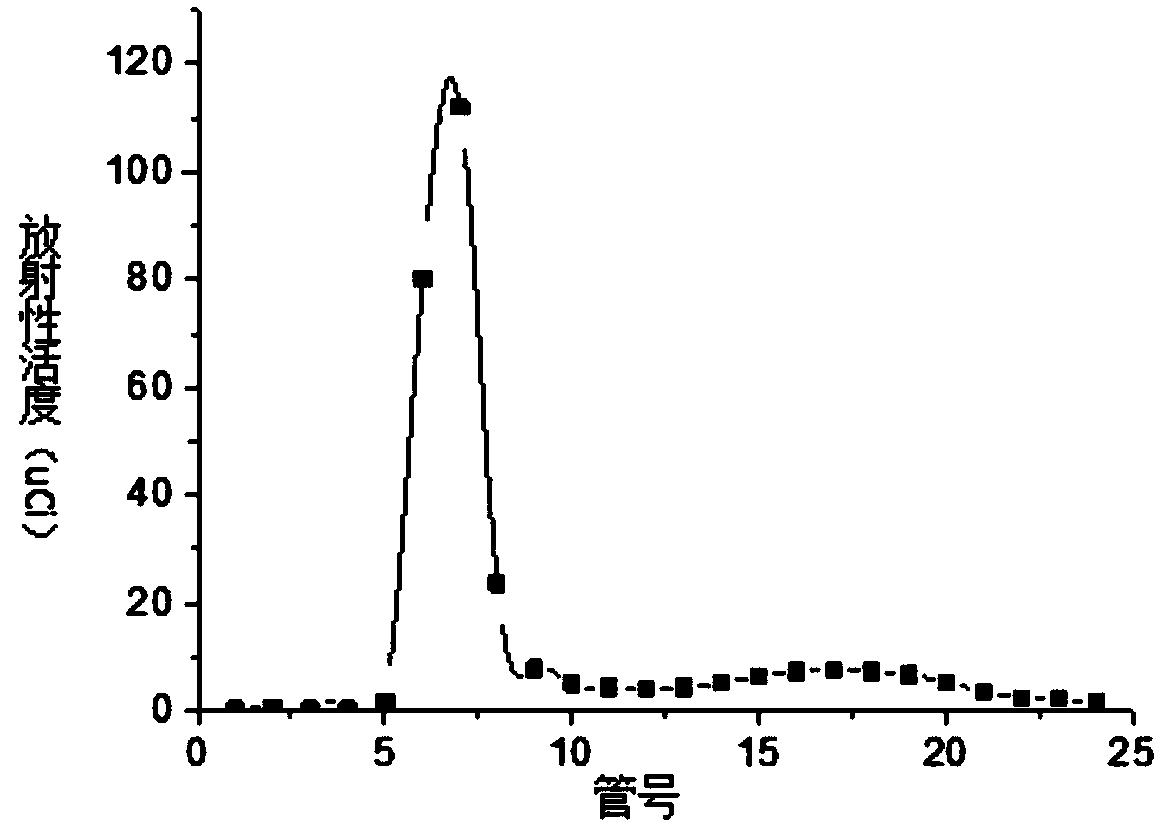
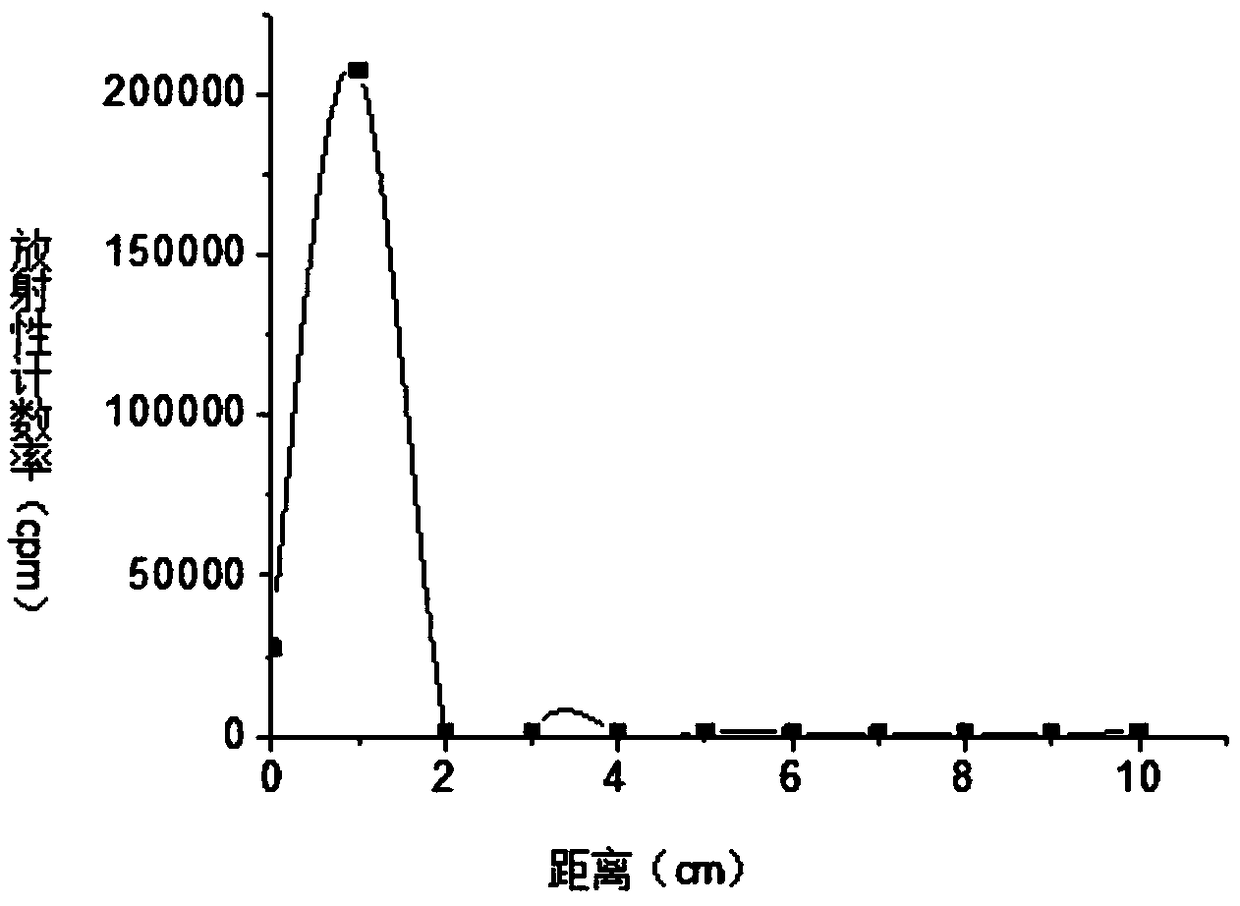
[0046] S4. Purify the reaction solution described in step S3 with a SephadexG-25 chromatography column, elute with a phospha...
Embodiment 2125I-F3
[0050] Example 2 125 I-F3 Stability Investigation
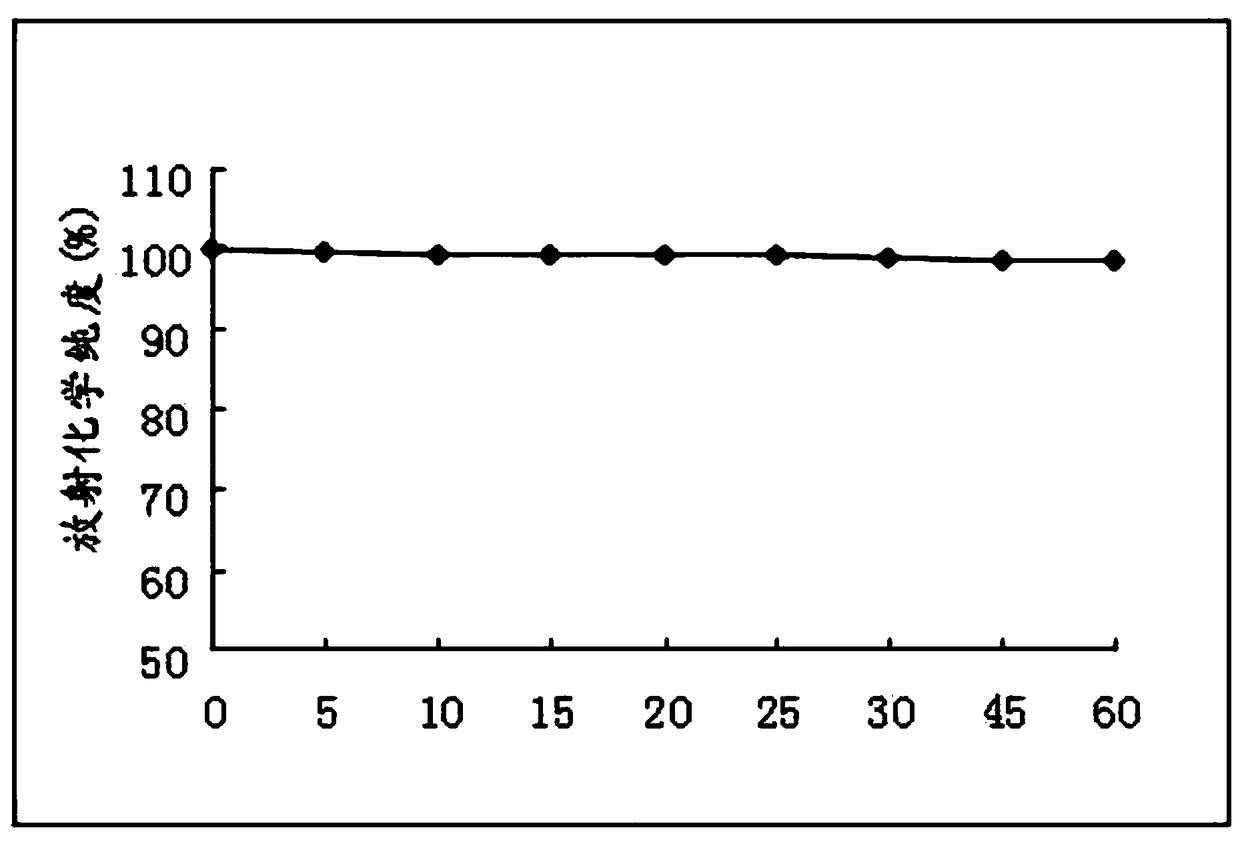
[0051] (1) take 125 I-F3, placed under the condition of 4°C for 60h, measured respectively after 0h, 5h, 10h, 15h, 20h, 25h, 30h, 45h, 60h, 125 The radiochemical purity of I-F3, see image 3 . Depend on image 3 The results show that the present invention provides 125 After I-F3 was stored at 4°C for 60 hours, the radiochemical purity was still as high as 98.25%.
[0052] (2) take 125 I-F3, placed under the condition of 25 ℃ for 24h, measured respectively after 2h, 4h, 8h, 12h, 16h, 20h, 24h, 125 The radiochemical purity of I-F3, see Figure 4 . Depend on Figure 4 The results show that the present invention provides 125 After I-F3 was stored at 25°C for 24 hours, the radiochemical purity was still as high as 98.01%.
[0053] (3) Take 50 μL of 125 I-F3 was added to 100 μL of normal saline and 100 μL of human plasma, and placed at a temperature of 37°C for 48 hours. After 10 minutes, 20 minutes, 30 minutes, 1 hour...
Embodiment 3125I-F3
[0054] Example 3 125 Inhibitory effect of I-F3 on the growth of MCF-7 cells in vitro
[0055] Take the MCF-7 cells (human breast cancer cells) in the logarithmic growth phase, adjust the cell concentration to 1×10 with RPMI1640 medium containing 10% volume fraction calf serum 5 / mL, seeded in a 96-well plate, 100 μL per well, and 6 parallel wells for each group. After inoculation, different concentrations of 125 I-F3 medium 10 μL / well, 125 The radioactivity of I-F3 was 1, 10, 37, 100, 200 and 500kBq / well respectively, and the control group Na 125 The I radioactivity is 1, 10, 37, 100, 200, 500kBq / well, and the blank group is an equal-volume culture medium without cells, placed at 37°C and 5% CO 2 After culturing in the incubator for 24 and 48 hours, add 10 μL / well of MTT (5 mg / mL), continue to incubate for 4 hours, then terminate the culture, add 10% SDS-HCl 100 μL / well, and act at 37°C for 12 hours. A wavelength of 570nm was selected, and the optical absorption value (OD...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com