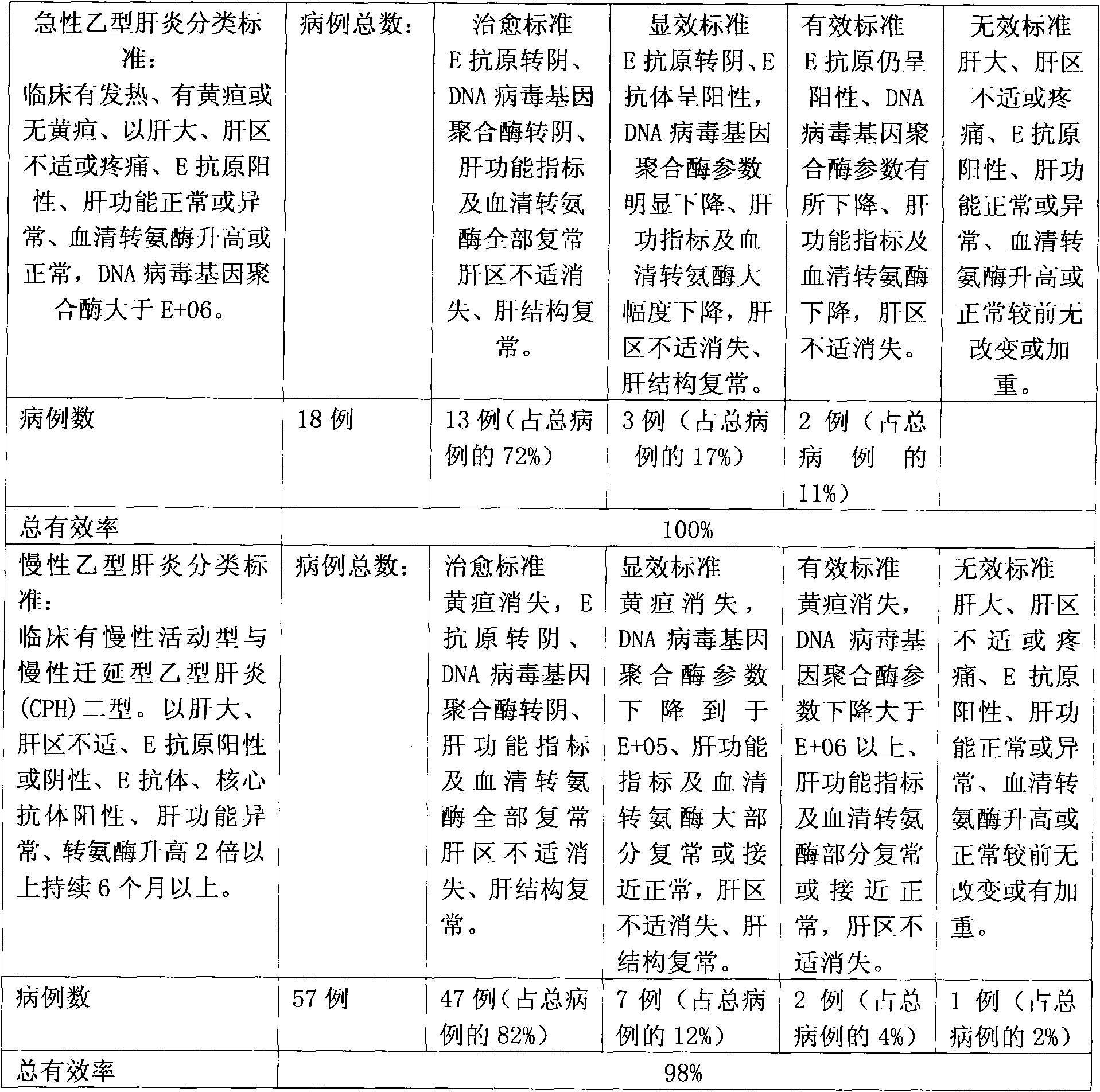
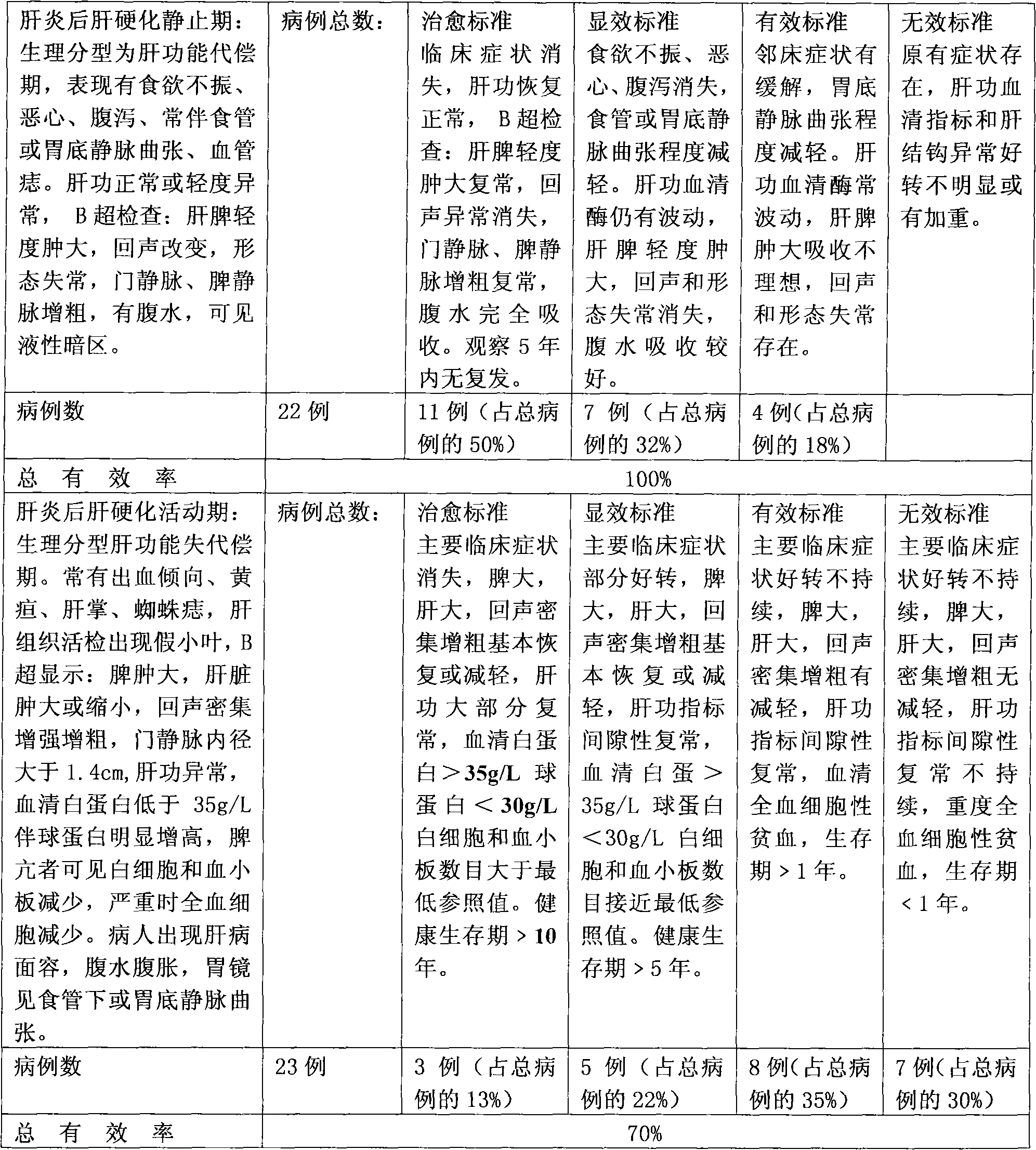
Pharmaceutical composition for treating hepatitis B and cirrhosis
A composition and drug technology, applied in the direction of drug combination, digestive system, pharmaceutical formula, etc., to achieve the effects of rapidly reducing liver function serum enzymes, inhibiting virus replication, and reducing liver fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 pharmaceutical composition pill of the present invention
[0025] Take 8g golden buckwheat, 5g black nightshade, 5g zedoary, 3g horsetail, 5g malan, 3g carrageenan, 5g fennel, 4g sophora flavescens, 4g capillary, 8g coix seed, 3g comfrey, after crushing, add appropriate auxiliary materials, Pan pills.
Embodiment 2
[0026] The preparation of embodiment 2 pharmaceutical composition decoction of the present invention
[0027] Take 8g golden buckwheat, 3g black nightshade, 2g zedoary, 2g horsetail, 3g malan, 3g carrageenan, 3g fennel, 3g flavescens, 3g capillary, 3g coix seed, 3g comfrey, add water and fry, combine the filtrate, Get decoction of the present invention.
Embodiment 3
[0028] Embodiment 3 Preparation of pharmaceutical composition formula granules of the present invention
[0029] Take 4g golden buckwheat, 5g black nightshade, 5g zedoary, 3g horsetail, 5g malan, 5g carrageenan, 5g fennel, 5g flavescens, 5g capillary, 8g coix seed, 5g comfrey, after crushing, add appropriate auxiliary materials, Pan pills. After respectively adding water for extraction, the filtrate is concentrated, and auxiliary materials are added to prepare formula granules respectively, thus obtaining the formula granules of the present invention. Mix before use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com