Hepatitis B virus e antigen quantitative detection kit, preparation method and detection method thereof
A technology for detection of hepatitis B virus and antigen, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of low sensitivity, limited application, narrow measurement range, etc., and achieve high detection sensitivity, facilitate promotion, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The hepatitis B virus e antigen detection kit of the present invention consists of hepatitis B e antigen calibrator, anti-HBe monoclonal antibody coated plate, europium-labeled anti-HBe monoclonal antibody, analysis buffer, fluorescence enhancement solution and concentrated The lotion is composed, and its specific preparation method is as follows:
[0024] 1. Preparation of hepatitis B e antigen calibrator
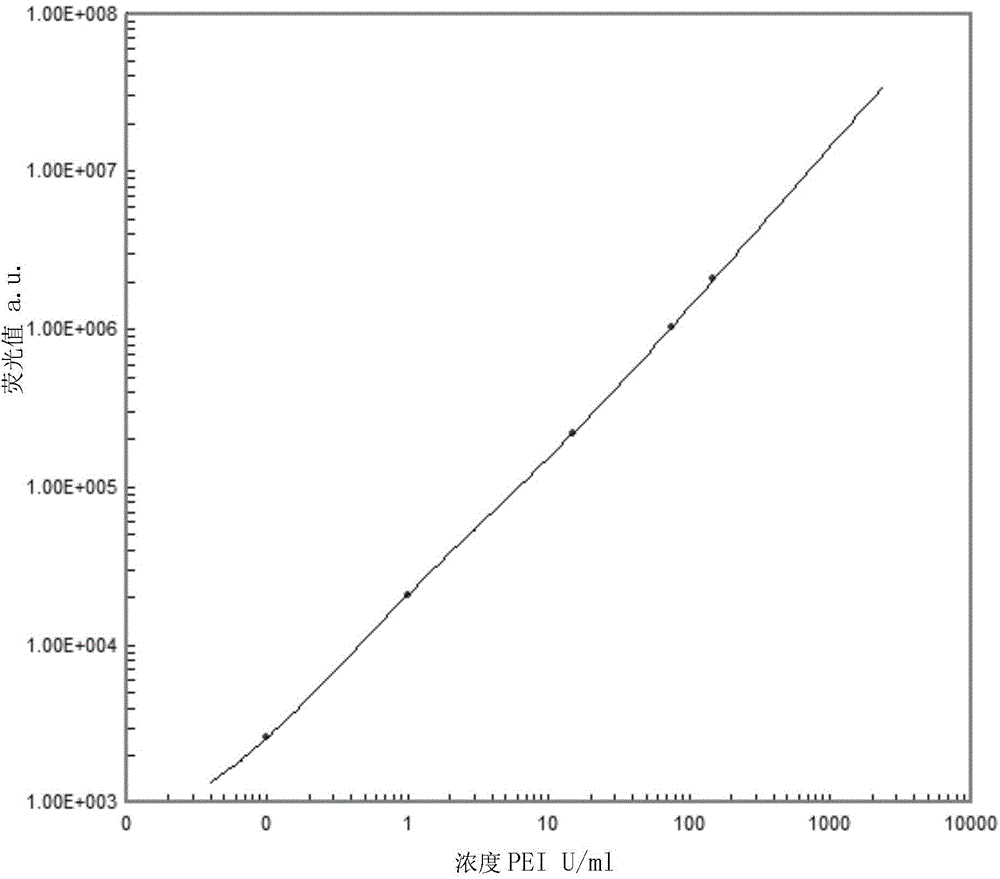
[0025] The linear series (concentration is 0, 0.1, 1.5, 15, 75, 100PEIU / ml) prepared by the PEIHBeAg82 reference material prepared by the Paul-Ehrlich-Institut institution in Germany is used to calibrate the HBeAg enterprise standard product prepared by positive plasma, and use the standardized The HBeAg enterprise standard product is used to calibrate the HBeAg recombinant antigen, and then diluted to 0, 0.1, 1.5, 15, 75, 150PEIU / ml HBeAg calibrator.
[0026] 2. Fabrication of Anti-HBe Monoclonal Coated Plates
[0027] Dilute the three anti-HBe monoclonal antibod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com