Hepatitis B e antibody magnetic particle chemiluminescent immunoassay kit and preparation method thereof
A chemiluminescence immunoassay and chemiluminescence technology, applied in the field of immunoassay, can solve the problems of false positive, complicated operation, low sensitivity, etc., and achieve the effect of large surface area and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Preparation of hepatitis B e antibody magnetic particle chemiluminescence immunoassay assay kit of the present invention
[0024] 1. Preparation of a mixture of hepatitis B e antibody-coated magnetic particles and horseradish peroxidase-labeled hepatitis B e antibody
[0025] 1. Preparation of hepatitis B e antibody-coated magnetic particles
[0026] Activate the magnetic particles with a particle size of 1-10 μm with glutaraldehyde, stir at room temperature, mix for 3 hours, apply a magnetic field, let stand for 20-25 minutes, pour out the supernatant, and use 0.01mol / L with a pH value of 7.4 Wash three times with phosphate buffer solution, and suspend with the solution, the concentration is 50-100 mg / mL; add 40-100 μg of hepatitis B e antibody to each ml of suspension, stir overnight at 4°C, add a magnetic field, and let it stand for 10-15 minutes , pour out the supernatant, and block with phosphate buffer (pH 7.2) containing 0.2% to 1.0% bovine serum al...
Embodiment 2
[0046] Embodiment 2 The using method of kit of the present invention
[0047] 1. Sample preparation
[0048] Collect patient serum for testing using correct medical methods.
[0049] 2. Detection method
[0050] 1. Before using this kit for experiments, it is necessary to take out the mixture of antibody-coated magnetic particles and horseradish peroxidase-labeled antibody, hepatitis B e antigen, calibrator / sample to be tested, and enzyme-labeled avidin at room temperature. Leave them for 15-30 minutes to allow them to balance to room temperature; and pay attention to adjust the incubator or water bath to 37°C; then, prepare a suitable micro-sampler and corresponding tips and check whether the chemiluminescence instrument is working normally.
[0051] 2. Number the reaction tubes, add 50 μL of serum samples or serial calibrator solutions to the reaction tubes, add 0NCU / mL, 2NCU / mL, 4NCU / mL, 8NCU / mL, 16NCU / mL, 32NCU / mL each 50 μL.
[0052] 3. Add 50 μL of the mixture of ant...
Embodiment 3
[0057] The methodological examination of the kit of the present invention of embodiment 3
[0058] The test kit prepared in Example 1 is tested according to the conventional manufacturing and testing procedures in the art, and the results are as follows:
[0059] 1. Determination of kit precision
[0060] (1) Calibrator precision experiment
[0061] Three batches of the kits prepared in Example 1 were taken for the precision test, and 10 kits were taken from each batch. Measure the 4NCU / mL HBeAb calibrator 5 times with the kit extracted in Example 1. The coefficient of variation was calculated, and the result showed that the coefficient of variation was between 4.6% and 7.7%.
[0062] 2. Determination of the accuracy of the kit
[0063] Detected with 4NCU / ml quality control serum of hepatitis B e antibody from clinical inspection center, and the deviation of the measured value was less than 10%.
[0064] 3. Kit specificity and sensitivity experiments
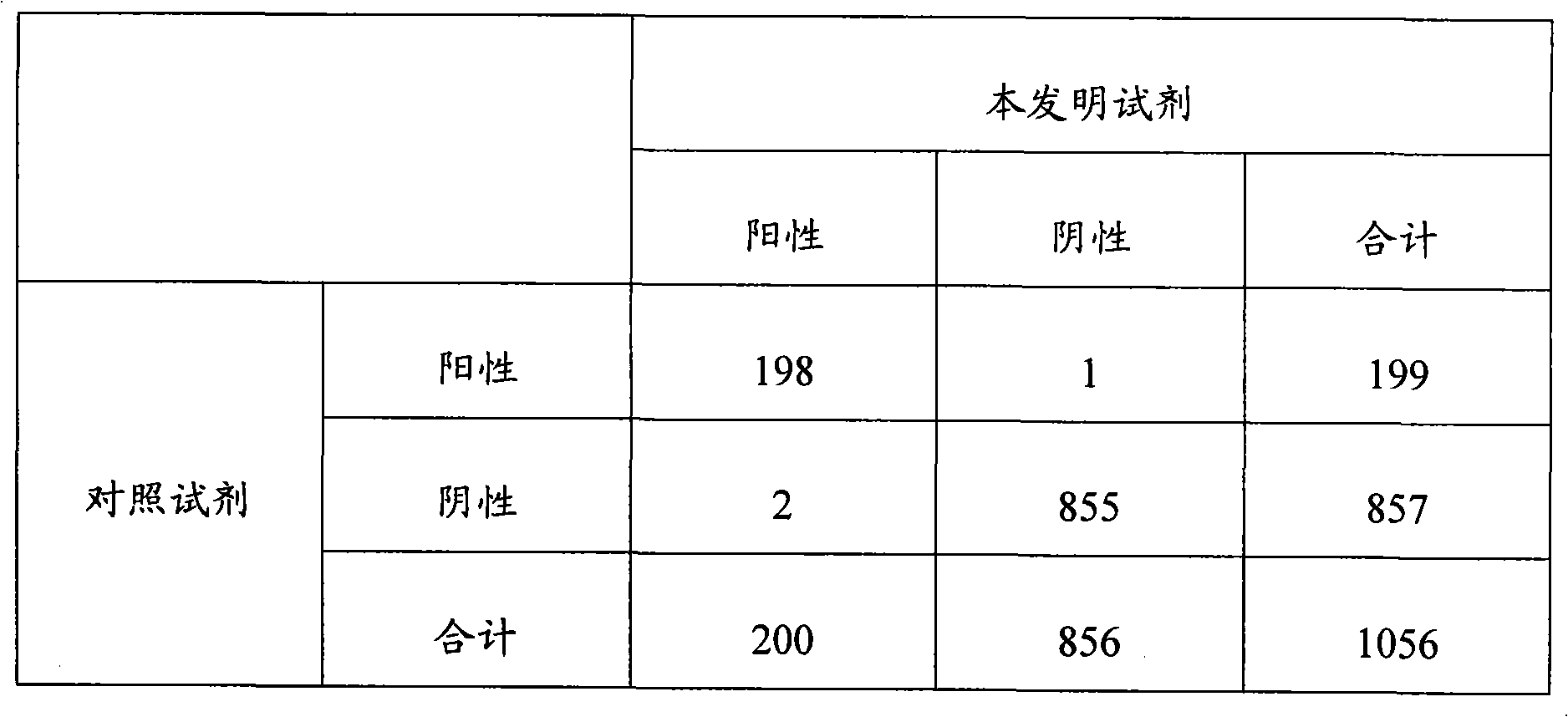
[0065] There is no...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com