Application of benzoyl silibinin for preparing medicaments for treating viral hepatitis B
A technology of silibinin and dimethoxybenzoyl, which is applied in the field of drugs for the treatment of hepatitis B virus infection, can solve the problems of no reported anti-HBV activity of flavonoid lignans, and facilitate large-scale production , Large-scale production of energy saving and emission reduction, and the effect of clear industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
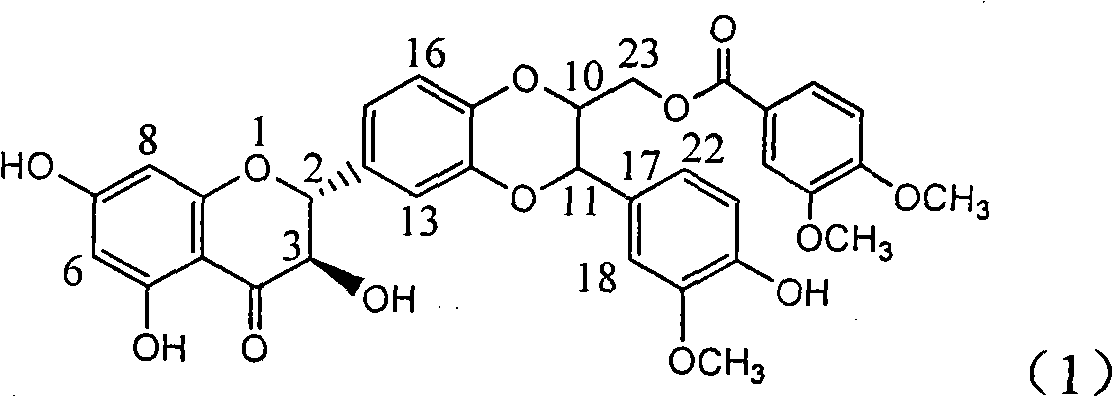
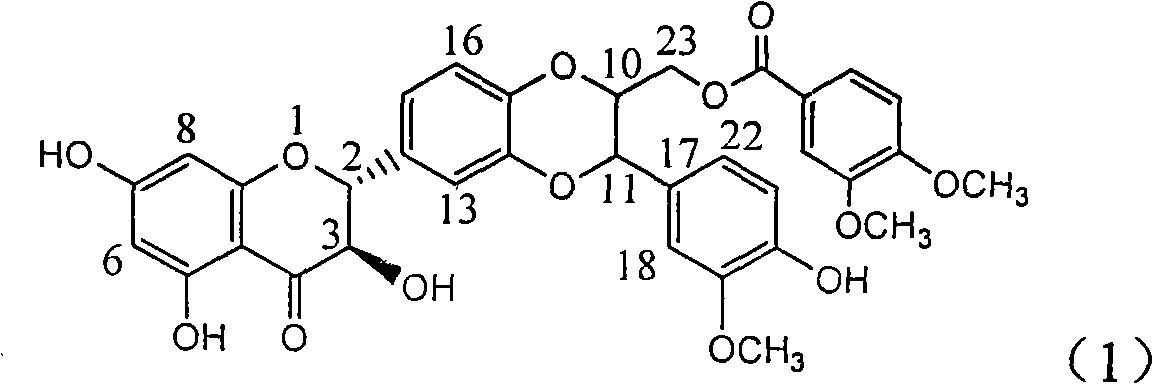
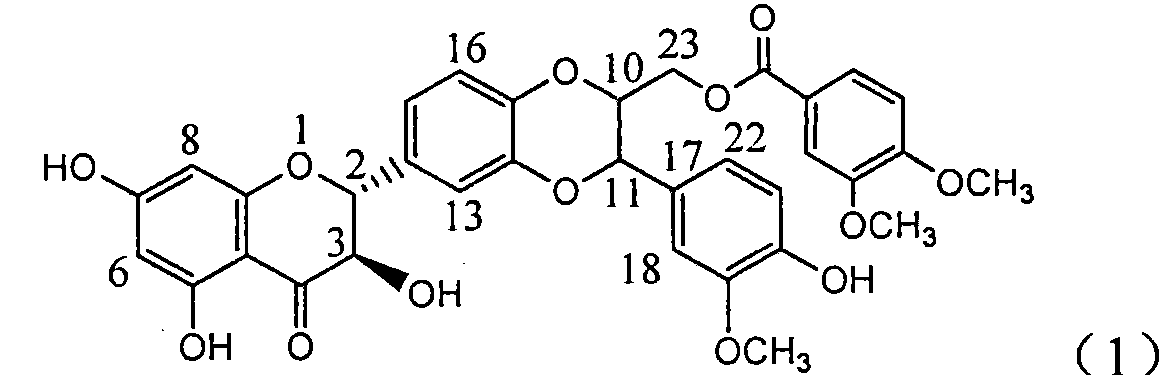
[0029] Example 1: Formula (1) compound (±)-3,4-dimethoxybenzoic acid [3-(4-hydroxyl-3-methoxyphenyl)-6-(2,3-dihydro-3,5, Preparation of 7-trihydroxy-4-oxo-1-benzopyran-2)-2,3-dihydro-1,4-benzodioxane-2]methyl ester
[0030] 1.1 Instruments and reagents:
[0031] The ultraviolet spectrum was measured with a Shimadzu UV-240 ultraviolet spectrophotometer; the hydrogen nuclear magnetic resonance spectrum 1 H-NMR is measured by INOVA type superconducting nuclear magnetic resonance spectrometer (VARIAN INOVA-400MHz) (tetramethylsilyl ether TMS is the internal standard); (100-200, 200-300 and 300-400 mesh) and silica gel GF254 (10-40 mesh) for thin-layer chromatography are all produced by Qingdao Ocean Chemical Factory; all reagents used are analytically pure, and the boiling range of petroleum ether is 60 -90°C; thin-layer preparative chromatography (PTLC) uses aluminum foil silica gel plates from Merck; column chromatography uses dextran gel Sephadex LH-20 from Amersham Pharmaci...
Embodiment 2
[0035] Example 2: Inhibitory Effect of Compound of Formula (1) on Hepatitis B Surface Antigen (HBsAg) Secreted by HepG2.2.15 Cells
[0036] 2.1 Cell culture:
[0037] HepG2.2.15 cells were cultured in DMEM medium containing 10% inactivated fetal bovine serum, 100 U / ml penicillin and 100 U / ml streptomycin, 100 μg / ml G418 at 37°C, 5% CO 2 , cultured in an incubator with 100% relative humidity.
[0038] 2.2 The inhibitory effect of the compound of formula (1) on HepG2.2.15 cell growth and HBsAg was measured by MTT method:
[0039] Take the HepG2.2.15 cells in the logarithmic growth phase, and dilute the cells to 1×10 with medium 5 cells / ml, seeded in 96-well cell culture plate, 100 μl per well, at 37°C, 5% CO 2 After cultivating in the incubator of 100% relative humidity for 24 hours, add the compound of formula (1) diluted with medium, the concentration is respectively 1000 microgram / ml, 200 microgram / ml, 40 microgram / ml and 8 microgram / ml, every hole 200 microliters, three ...
Embodiment 3
[0048] Example 3: Inhibitory effect of compound of formula (1) on hepatitis B e antigen (HBeAg) secreted by HepG2.2.15 cells
[0049] 3.1 Cell culture: the method is the same as in Example 2.
[0050] 3.2 Determination of the inhibitory effect of the compound of formula (1) on the growth of HepG2.2.15 cells by MTT method: the method is the same as in Example 2.
[0051] 3.3 Determination of the inhibitory effect of the compound on hepatitis B e antigen (HBeAg): take the HepG2.2.15 cells in the logarithmic growth phase, and dilute the cells to 1 × 10 with the medium 5 / ml, seeded in 96-well cell culture plate, 100ml per well, at 37°C, 5% CO 2 After culturing in an incubator with 100% relative humidity for 24 hours, add samples diluted with culture medium at concentrations of 20 μg / ml, 4 μg / ml and 0.8 μg / ml, 200 μl per well, and set three concentrations for each Multiple wells were placed at 37°C, 5% CO 2 , cultivated in an incubator with 100% relative humidity, change the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com