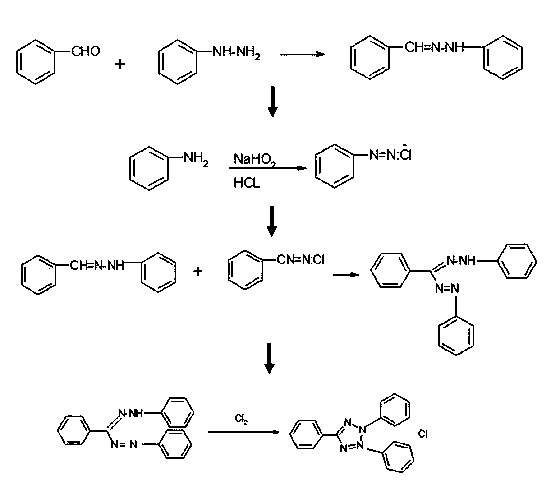
Synthesis method of chloridized-2,3,5-triphenyl tetrazolium chloride
A technology of triphenyltetrazolium and synthesis method, applied in the direction of organic chemistry, etc., to achieve the effects of increased yield, easy source of raw materials, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (4) Synthesis of 2,3,5-triphenyltetrazolium chloride: put the 2,3,5-triphenylformazan obtained in step (3) into a 100ml three-necked flask, and then add 60ml of methanol , turn on the stirring device, cool down to below 0°C with an ice bath, and slowly introduce Cl into the solution 2 The gas is saturated, the reaction process is obviously exothermic, and the Cl is controlled to 2 Speed, the temperature during the reaction should not exceed 15°C. With Cl 2 The feeding of the flask, the black crystals suspended in the flask gradually decreased, and the solution gradually turned brown, and when the solution was clear and free of suspended solids, stop passing Cl 2 , the reaction was stirred at room temperature for 2 h. After the reaction was completed, the solvent was evaporated under reduced pressure to obtain 6.1 g of a yellow-brown crude product with a yield of 61%. The solid was refluxed with chloroform, decolorized with activated carbon, filtered with suction, th...
Embodiment 2
[0030] (4) Synthesis of 2,3,5-triphenyltetrazolium chloride: put the 2,3,5-triphenylformazan obtained in step (3) into a 250ml three-necked flask, and then add 100ml of methanol , turn on the stirring device, cool down to below 0°C with an ice bath, and slowly introduce Cl into the solution 2 The gas is saturated, the reaction process is obviously exothermic, and the Cl is controlled to 2 Speed, the temperature during the reaction should not exceed 15°C. With Cl 2 The feeding of the flask, the black crystals suspended in the flask gradually decreased, and the solution gradually turned brown, and when the solution was clear and free of suspended solids, stop passing Cl 2 , the reaction was stirred at room temperature for 2 h. After the reaction was completed, the solvent was evaporated under reduced pressure to obtain 12.6 g of a yellow-brown crude product with a yield of 63%. The solid was refluxed with chloroform, decolorized with activated carbon, filtered with suction, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com