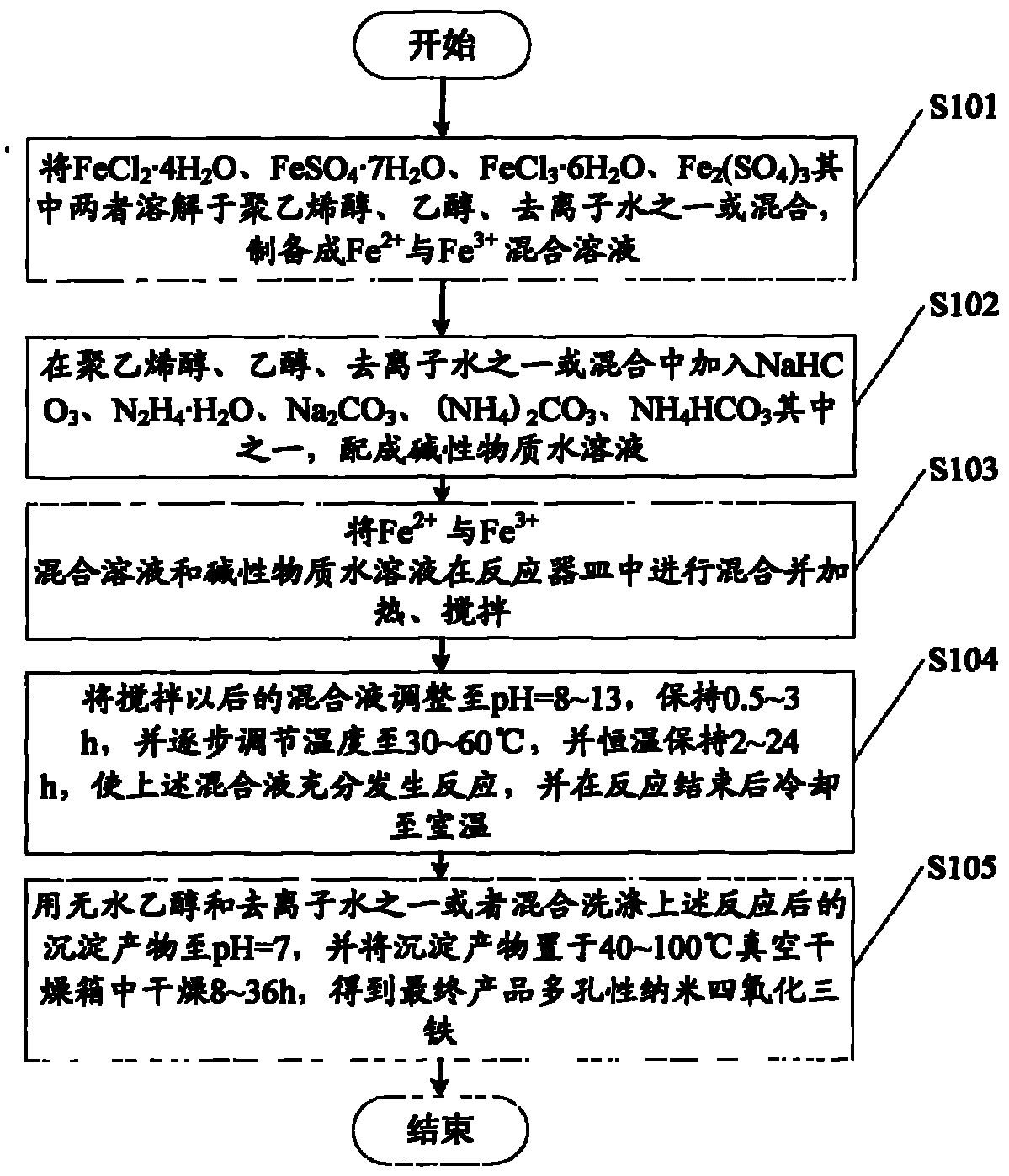
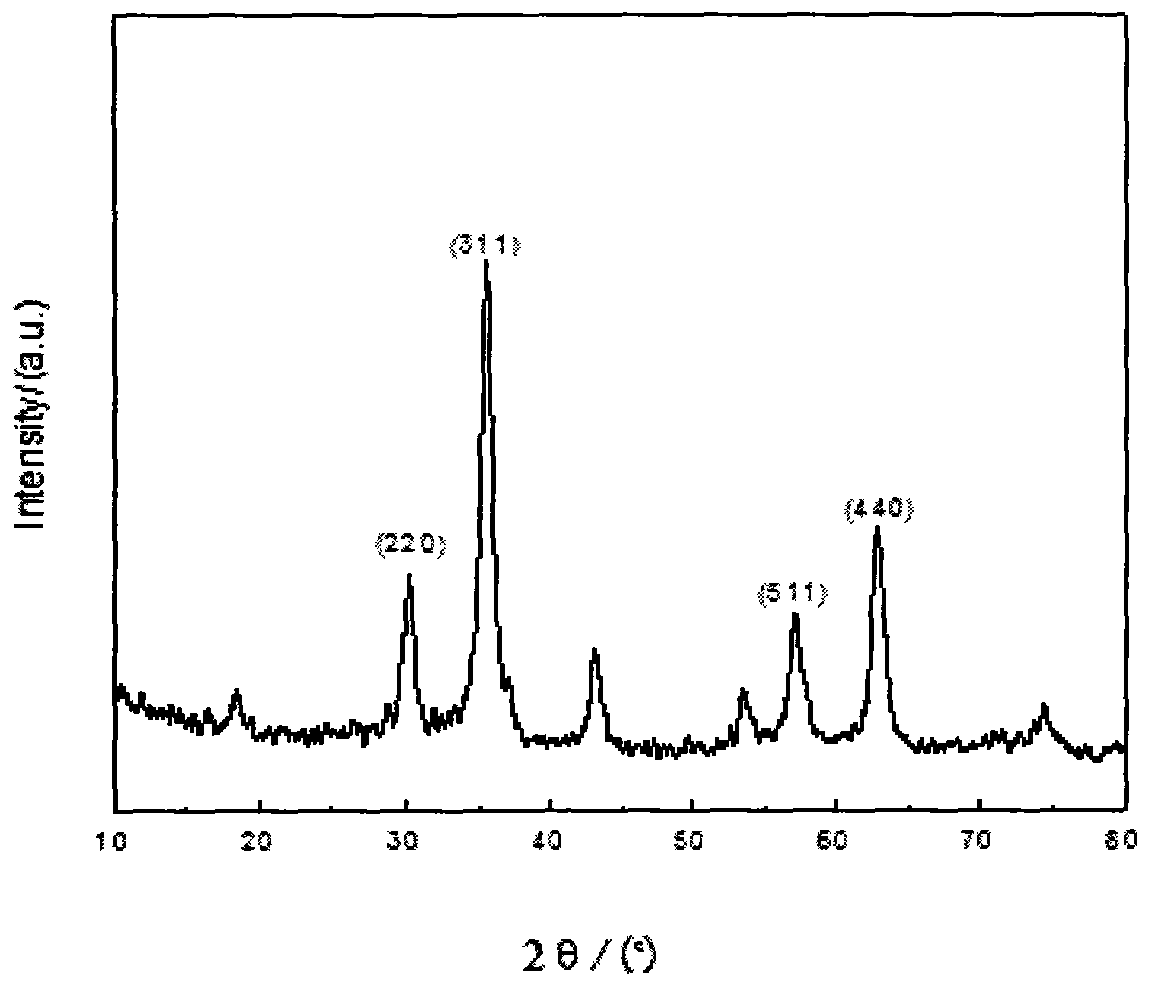
Method for preparing porous nano ferroferric oxide
A technology of ferroferric oxide and porosity, which is applied in the chemical field, can solve the problems of increasing industrialization costs, increasing requirements for reaction equipment, unfavorable industrialization promotion, etc., and achieves low cost, convenient and easy-to-obtain raw material sources, and low threshold for industrialized production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0047] Step 201: Massage Erbi〔n(Fe 2+ ): n(Fe 3+ )] = 1~3 Weigh Fe 2+ (FeCl 2 ·4H 2 O, FeSO 4 ·7H 2 O) and Fe 3+ (FeCl 3 ·6H 2 O, Fe 2 (SO4) 3 ), dissolved in 50~300mL polyvinyl alcohol / ethanol / deionized water to prepare Fe 2+ With Fe 3+ mixture;
[0048] Step 202: configure 0.5~10mol / L of precipitation agent NaHCO 3 / Na 2 CO 3 / NH 4 HCO 3 Alkaline substances such as polyvinyl alcohol / ethanol / deionized aqueous solution that are prone to iron salt formation and precipitation.
[0049] Step 203: Add NaHCO to 100 mL of deionized water in a 500 mL three-necked flask 3 , Na 2 CO 3 , (NH 4 ) 2 CO 3 , NH 4 HCO 3 One of the inorganic compounds that can easily decompose and generate non-oxygen. Heat to 40°C and keep for 20 minutes. This step is to remove oxygen in the reaction vessel;
[0050] Step 204: Transfer 201 and 202 to a three-necked flask, and under the conditions of constant temperature of 70°C and rapid mechanical stirring of 200r / min, drop the liquid to a certain pH=8, keep it for ...
Embodiment 3
[0058] Step 301: Molar ratio [n(Fe 2+ ): n(Fe 3+ )] = 1~3 Weigh Fe 2+ (FeCl 2 ·4H 2 O, FeSO 4 ·7H 2 O) and Fe 3+ (FeCl 3 ·6H 2 O, Fe 2 (SO4) 3 ), dissolved in 100mL polyvinyl alcohol / ethanol / deionized water to prepare Fe 2+ With Fe 3+ mixture;
[0059] Step 302: configure 0.5~10mol / L NaHCO 3 / Na 2 CO 3 / NH 4 HCO 3 Alkaline substances such as polyvinyl alcohol / ethanol / deionized aqueous solution that are prone to iron salt formation and precipitation.
[0060] Step 303: Add NaHCO to 100 mL of deionized water in a 500 mL three-neck flask 3 , N 2 H 4 ·H 2 O, Na 2 CO 3 , (NH 4 ) 2 CO 3 , NH 4 HCO 3 One of the inorganic compounds that can easily decompose and generate non-oxygen, heat to 80°C and keep it for 20 minutes;
[0061] Step 304: Transfer 301 and 302 to a three-necked flask, and under the condition of temperature rising to 60°C and constant temperature, 500r / min rapid mechanical stirring, drop the liquid to a certain pH=10, keep it for 2h, and gradually reduce the temperature to 30...
Embodiment 4
[0064] Step 401: Molar ratio [n(Fe 2+ ): n(Fe 3+ )] = 1~3 Weigh Fe 2+ (FeCl 2 ·4H 2 O, FeSO 4 ·7H 2 O) and Fe 3+ (FeCl 3 ·6H 2 O, Fe 2 (SO4) 3 ), dissolved in 300mL polyvinyl alcohol / ethanol / deionized water to prepare Fe 2+ With Fe 3+ mixture;
[0065] Step 402: configure 10 mol / L precipitant NaHCO 3 / Na 2 CO 3 / NH 4 HCO 3 Alkaline substances such as polyvinyl alcohol / ethanol / deionized aqueous solution that are prone to iron salt formation and precipitation.
[0066] Step 403: Add NaHCO to 100mL deionized water in a 500mL three-neck flask 3 , N 2 H 4 ·H 2 O, Na 2 CO 3 , (NH 4 ) 2 CO 3 , NH 4 HCO 3 One of the inorganic compounds that can easily decompose and generate non-oxygen, heat to 80°C and keep it for 40 minutes;
[0067] Step 404: Transfer 401 and 402 to a three-necked flask, and under the condition of increasing the temperature to 80°C and constant temperature, 200r / min rapid mechanical stirring, drip the liquid to a certain pH=12, keep it for 3h, and gradually reduce the tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com