Rifaximin medicine composition and preparation method thereof
A technology of rifaximin and its composition, which is applied in the field of pharmaceutical composition and its preparation, can solve the problem that the pathogenesis of hepatic encephalopathy is not completely clear, and achieve the effect of rapid disintegration, uniform dispersion and fast dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] Specific embodiments of the present invention will be described in detail below.
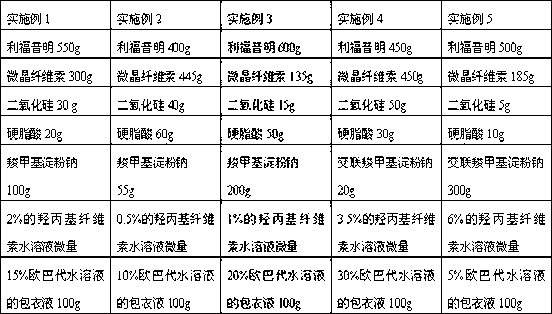
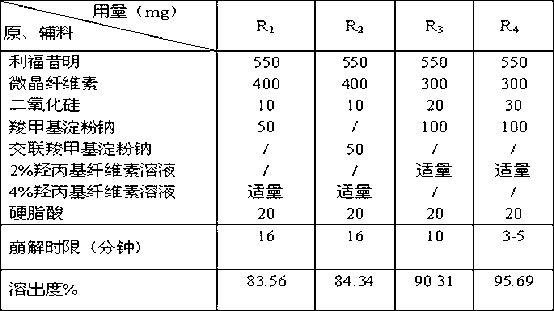
[0021] The pharmaceutical composition of rifaximin provided by the present invention comprises the following components: 40%-60% of rifaximin by mass percentage, 10%-50% of microcrystalline cellulose, 1%-30% of carboxyl Sodium methyl starch or cross-linked sodium carboxymethyl starch, 0.5%-6% silicon dioxide, 0.5%-6% stearic acid, and a trace amount of hydroxypropyl cellulose aqueous solution with a mass concentration of 0.5%-6% . Preferably 45%-55% rifaximin, 20%-40% microcrystalline cellulose, 5%-20% sodium carboxymethyl starch or cross-linked sodium carboxymethyl starch, 1%-5% Silicon dioxide, 1%-3% stearic acid, and a trace amount of hydroxypropyl cellulose aqueous solution with a mass concentration of 1%-3%. Most preferably be 55% rifaximin by mass percentage, 30% microcrystalline cellulose, 10% sodium carboxymethyl starch, 3% silicon dioxide, 2% stearic acid, and mass concentratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com