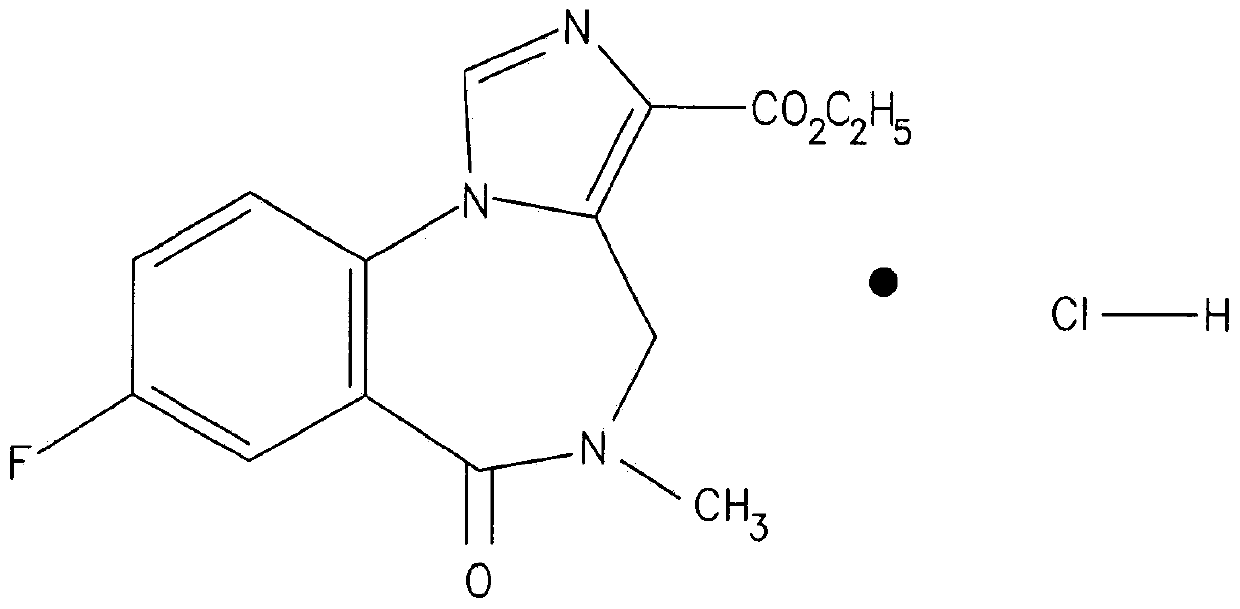
Flumazenil complexes, compositions comprising same and uses thereof
一种氟马西尼、络合物的技术,应用在可溶性络合物领域,能够解决未满足等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Example 1 . Preparations of Flumazenil Salts and Flumazenil Complexes Containing 1% Flumazenil
[0137] Formulations containing 1% flumazenil in the form of flumazenil salts or flumazenil complexes are presented in the table below. All formulations were evaluated for appearance and only clear solutions, ie clear solutions in which no precipitate ('ppt') or slight precipitate ('sl-ppt') or few particles ('part') formed, were tested for pH.
[0138] First, the composition comprising niacinamide and meglumine as complexing agents resulted in a clear solution. After 12 hours, a precipitate formed in the formulation containing meglumine. Formulation No. 2 containing 4.5% niacinamide showed less precipitate (designated "ppt" in the table) after 12 hours compared to formulation No. 1 containing 1.5% niacinamide (Table 1). This result suggests that high niacinamide concentrations may be preferred, although not necessary to achieve the invention. The appropriate pH of the c...
Embodiment 2
[0147] Example 2 .Preparation of flumazenil (1.2% w / w and 1.5% w / w) nicotinamide complex
[0148] Formulations comprising 1.2% w / w to 1.5% w / w of flumazenil in the form of flumazenil complexes containing niacinamide were prepared and are presented in the table below.
[0149] Table 5. Flumazenil Formulations Containing Flumazenil Niacinamide Complex (% w / w)
[0150]
Embodiment 3
[0151] Example 3 . Preparation of flumazenil nicotinamide complex and menthol
[0152]A formulation containing 1.2% w / w flumazenil based on formulation 16B (Table 6, above) was prepared using menthol and is presented in the table below.
[0153] Table 6. Flumazenil Formulations Containing 1.2 Flumazenil (% w / w) and Menthol
[0154]
[0155] The results were similar to those obtained in the absence of menthol (see Tables 4-5, above), suggesting that menthol is not necessary to achieve the invention, especially the flumazenil salts or flumazenil complexes improved solubility.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com