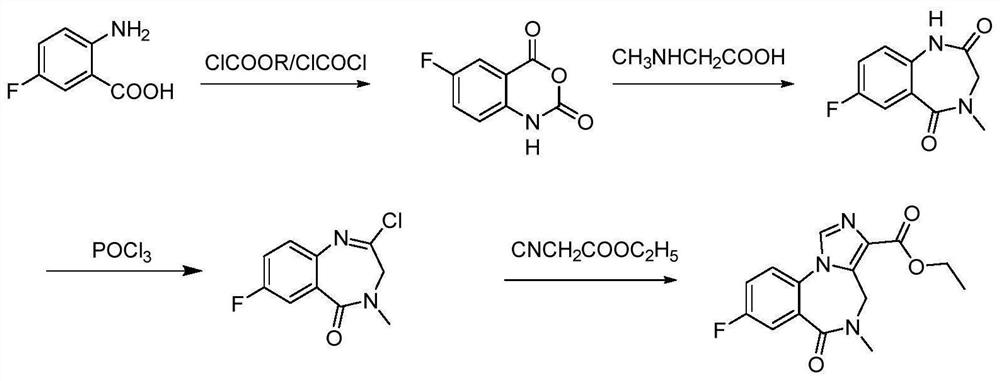
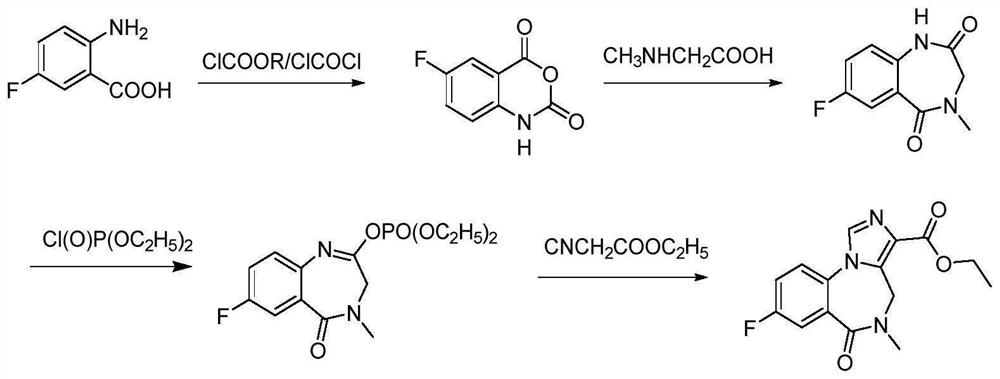
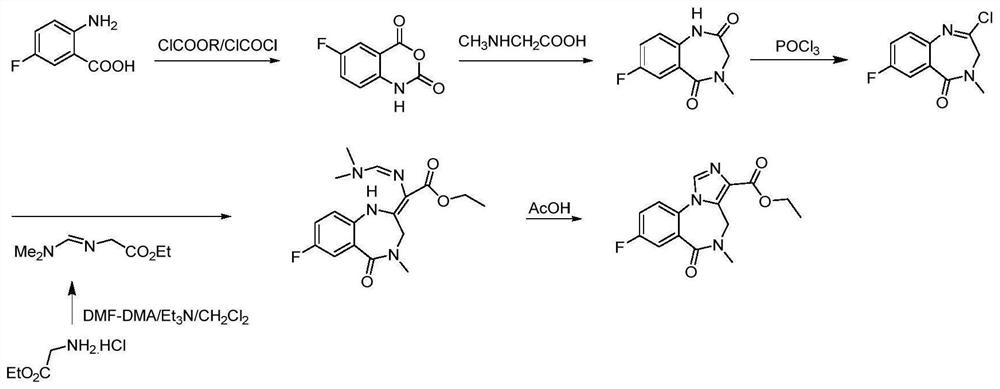
Preparation method of flumazenil
A technology of flumazenil and condensation reaction, which is applied in the field of drug synthesis, can solve problems such as water source, environmental pollution, cumbersome paths, and reaction failures, and achieve the effects of shortening the reaction path, reducing environmental risks, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] The preparation of embodiment 1 flumazenil
[0088] (1) Preparation of N-(5-fluoro-2-nitrobenzoyl)-N-methylglycine methyl ester
[0089]
[0090] Weigh 5-fluoro-2-nitrobenzoic acid (1.0g, 5.4mmol, CAS No.320-98-9; Anaiji chemical), 1-ethyl-(3-dimethylaminopropyl) carbon Imide hydrochloride (1.2g, 6.5mmol; EDCI, CAS No.: 25952-53-8, Anaiji Chemical), 1-hydroxybenzotriazole (0.87g, 6.5mmol; CAS No: 2592 -95-2, Anaiji Chemicals) was placed in a reaction bottle (100mL), added dichloromethane (20mL) and stirred to dissolve, and activated at room temperature for 30min; separately weighed sarcosine methyl ester hydrochloride (0.75g, 5.4mmol , CAS No.13515-93-0, Anaiji Chemical), triethylamine (1.0g, 10.8mmol, Sinopharm Group) were dissolved in dichloromethane (10mL), added to the reaction flask in sequence, and reacted at room temperature for 12h. Sampling by TLC to detect the reaction, the raw material point completely disappeared, and the reaction was terminated. Quenc...
Embodiment 2
[0104] (1) Same as Example 1, except that the base used is replaced by 4-dimethylaminopyridine (DMAP), and the solvent is replaced by N,N-dimethylformamide (DMF); wherein, 5-fluoro-2 - Nitrobenzoic acid: sarcosine methyl ester hydrochloride: 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI): 4-dimethylaminopyridine (4 -DMAP) molar ratio is 1:1:1:2, 5-fluoro-2-nitrobenzoic acid:N,N-dimethylformamide (DMF) is 1:20g / mL, reaction temperature is 35~ 40°C, the time is 24h; the yield of the obtained product is 80%.
[0105] (2) Same as Example 1, the difference is: the solvent is replaced by methanol; wherein, the quality of Raney nickel is the quality of N-(5-fluoro-2-nitrobenzoyl)-N-methylglycine methyl ester 5%, N-(5-fluoro-2-nitrobenzoyl)-N-methylglycine methyl ester: methanol is 1:20g / mL; the product yield is 75%;
[0106] (3) 7-fluoro-3,4-dihydro-4-methyl-1H-[1,4]benzodiazepine-2,5-dione (3.48g, 16.7mmol), N,N - Dimethylformamide (7.0mL), phosphorus oxychlori...
Embodiment 3
[0110] (1) Same as Example 1, except that the base used is replaced by 4-dimethylaminopyridine (4-DMAP); wherein, 5-fluoro-2-nitrobenzoic acid: methyl sarcosine hydrochloride: 1-Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI): 4-DMAP in a molar ratio of 1:1:1:2; 5-fluoro-2-nitro Benzoic acid: dichloromethane ratio is 1:20g / mL, reaction temperature is 37±2°C, time is 24h; the yield of the obtained product is 85%.
[0111] (2) with embodiment 1, difference is: the quality of Raney nickel is 15% of N-(5-fluoro-2-nitrobenzoyl)-N-methylglycine methyl ester quality; Embodiment 1 Methanol / purified water (v:v=1:3) in methanol / purified water (v:v=1:1) is replaced by methanol / purified water (v:v=1:1); Change " 120 ℃ of reaction 8h " in embodiment 1 to " 90 ℃ of reaction 8h "; The resulting product yield is 83%.
[0112] (3) With embodiment 2, product yield is 90%.
[0113] (4) With embodiment 2, the product yield obtained is 37%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com