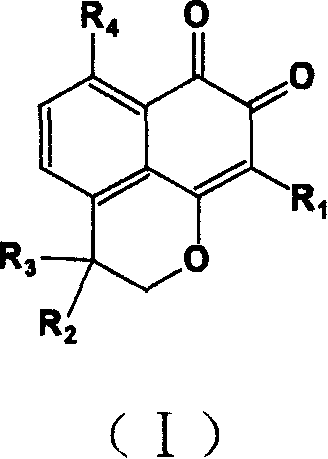
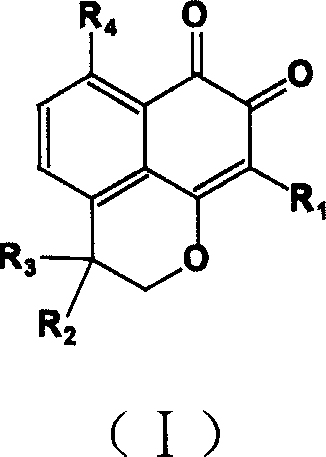
Compound containing structure of o-naphthaquinone and application
A compound, the technology of o-naphthoquinone, which is applied in the field of pharmaceutical compounds and its preparation, can solve problems such as unsatisfactory effects and achieve the effect of using a small dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
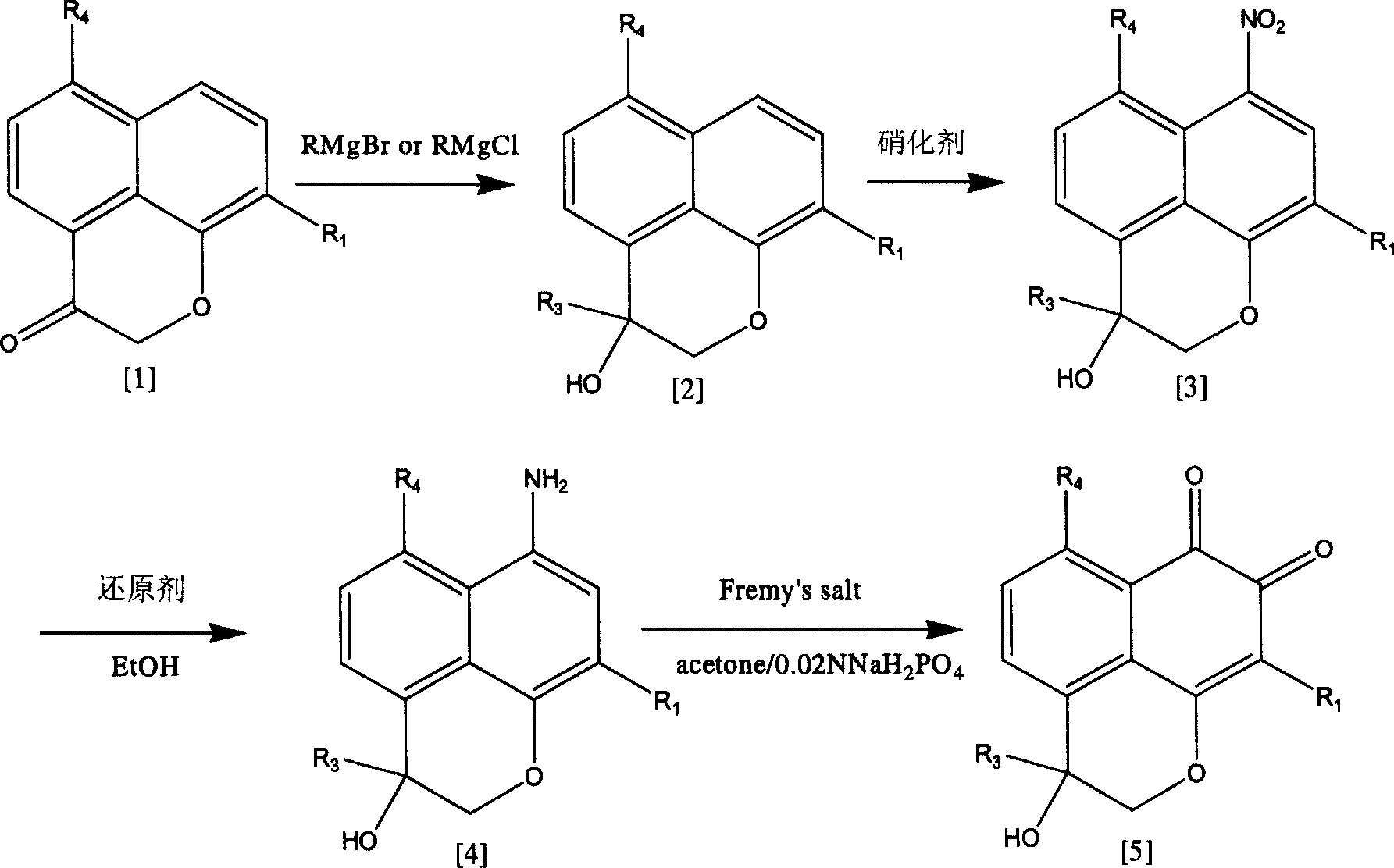
[0043] Preparation of 3-methyl-3-hydroxy-9-chloro-2,3-dihydronaphtho[1,8-b,c]pyran-7,8-dione:
[0044] Compound 9-chloronaphtho[1,8-b,c]pyran-3(2H)-one 7g was dissolved in 200ml of anhydrous ether, and the anhydrous ether of Grignard reagent MeMgI (100-150mmol) prepared in advance Put 70ml of the solution in a dropping funnel, add it in a water bath for 1.5-2 hours, stir at room temperature for 30 minutes, pour the reaction solution into 250ml of ice water solution, extract with chloroform, wash with water, dry over anhydrous sodium sulfate, and concentrate to obtain a brown-red viscous liquid 6.4g. Dissolve 6.4g of the product in 80-100ml of acetic anhydride, quickly add 8.5g of copper nitrate trihydrate, stir at room temperature for 15 minutes, add ice water to terminate the reaction, extract with ether, wash with aqueous sodium carbonate solution, then wash with water, anhydrous sodium sulfate Drying, concentration, silica gel column separation and purification, and chloro...
Embodiment 2
[0070] Preparation of 3-methyl-3-hydroxy-9-bromo-2,3-dihydronaphtho[1,8-b,c]pyran-7,8-dione:
[0071] Adopt 9-bromonaphtho[1,8-b,c]pyran-3(2H)-one as raw material, adopt the same method as Example 1, just adopt Na 2 S 2 o 4 As a reducing agent, 3-methyl-9-bromonaphtho[1,8-b,c]pyran-7,8-dione can be obtained.
[0072] Adopting 9-bromonaphtho[1,8-b,c]pyran-3(2H)-ketone is that raw material reacts with different Grignard reagents, adopts the method identical with embodiment 1 and 2 to also can obtain:
[0073] 1. 3-Propyl-3-hydroxy-9-bromo-2,3 dihydronaphtho[1,8-b,c]pyran-7,8-dione
[0074] 2. 3-Hexyl-3-hydroxy-9-bromo-2,3 dihydronaphtho[1,8-b,c]pyran-7,8-dione
[0075] 3. 3-allyl-3-hydroxy-9-bromo-2,3 dihydronaphtho[1,8-b,c]pyran-7,8-dione
Embodiment 3
[0077] Preparation of 3-methyl-9-chloro-2,3-dihydronaphtho[1,8-b,c]pyran-7,8-dione:
[0078] Using the compound 9-chloronaphtho[1,8-b,c]pyran-3(2H)-one as raw material, adopt a method similar to that of Example 1, except that 9-chloro-3-methyl-2, The one-step nitration reaction time of 3-dihydronaphtho[1,8-b,c]pyran-3-ol with acetic anhydride and copper nitrate trihydrate was extended to 1 hour to obtain 9-chloro-7-nitro-3 -Methyl-2,3-dihydronaphtho[1,8-b,c]pyran-3-ol acetate, other operations are the same as in Example 1, and finally obtain orange-red solid 3-methyl-9 -Chloro-2,3-dihydronaphtho[1,8-b,c]pyran-7,8-dione.
[0079] In the same way, you can also get:
[0080] 3-Propyl-9-chloro-2,3-dihydronaphtho[1,8-b,c]pyran-7,8-dione
[0081] 3-Benzyl-9-chloro-2,3-dihydronaphtho[1,8-b,c]pyran-7,8-dione
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com