Application of CLEC1B (C-type lectin domain family 1 member B) genes on diagnosis and treatment of bile duct carcinoma
A cholangiocarcinoma and gene technology, applied in the direction of gene therapy, medical preparations containing active ingredients, biochemical equipment and methods, etc., can solve the problems of low radical cure rate, inability to apply early diagnosis of cholangiocarcinoma, lack of diagnostic methods, etc. , to achieve early diagnosis, timely gene diagnosis, and reduce mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Screening of gene markers associated with cholangiocarcinoma
[0052] 1. Sample collection
[0053] Eight samples of normal bile duct tissue and cholangiocarcinoma tissue were collected. The above samples are surgical resection specimens of patients with cholangiocarcinoma, and all the above samples were obtained with the consent of the organizational ethics committee.
[0054] 2. Preparation of RNA samples (operated using QIAGEN tissue RNA extraction kit)
[0055] 1) Tissue extraction
[0056] In a clean area with less RNase interference, use a mortar containing an appropriate amount of liquid nitrogen to weigh about 20 mg of an isolated lung adenocarcinoma tissue sample, grind it to powder with a pestle, and then transfer the sample to a 2 ml RNase-free in a centrifuge tube. Add 300 μl lysate, place in a homogenizer, grind thoroughly for 1-5min, centrifuge at 12000g, 4°C for 10min, and transfer the supernatant to a new 1.5ml centrifuge tube. Add 600 μl ...
Embodiment 2
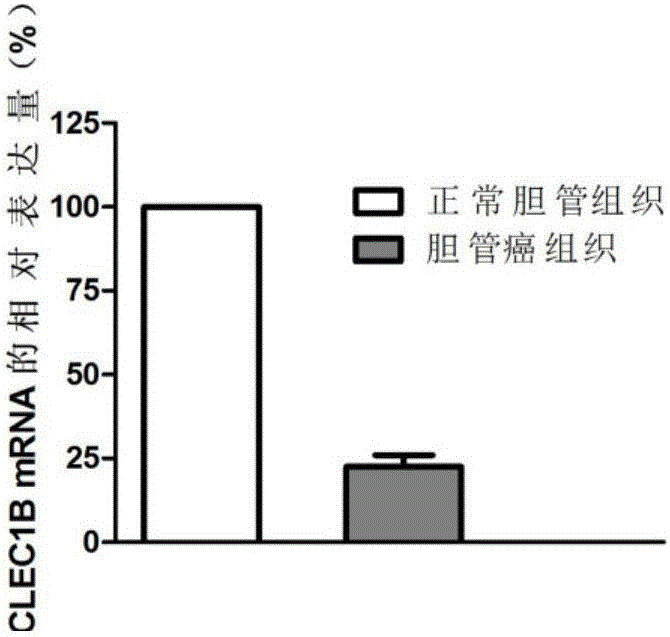
[0074] Example 2 QPCR sequencing verification of differential expression of CLEC1B gene
[0075] 1. Large-sample QPCR verification of differential expression of CLEC1B gene. According to the sample collection method in Example 1, 80 cases of cholangiocarcinoma tissue and 80 cases of normal bile duct tissue were selected.
[0076] 2. The specific operation steps of QPCR are as follows:
[0077] (1) RNA extraction
[0078] After collecting the samples, freeze them in liquid nitrogen. After taking them out, put the tissue into a pre-cooled mortar for grinding. After the tissue sample is powdered:
[0079] 1) Add Trizol and place at room temperature for 5 minutes;
[0080] 2) Add 0.2ml of chloroform, vibrate the centrifuge tube vigorously, mix well, and place it at room temperature for 5-10min;
[0081] 3) Centrifuge at 12000rpm for 15min, transfer the upper aqueous phase to another new centrifuge tube (be careful not to absorb the protein material between the two aqueous phas...
Embodiment 3
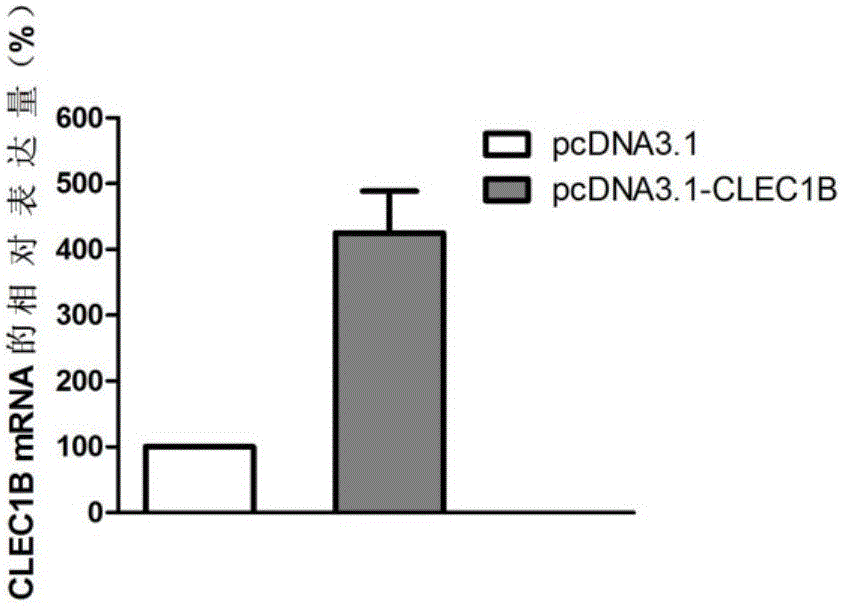
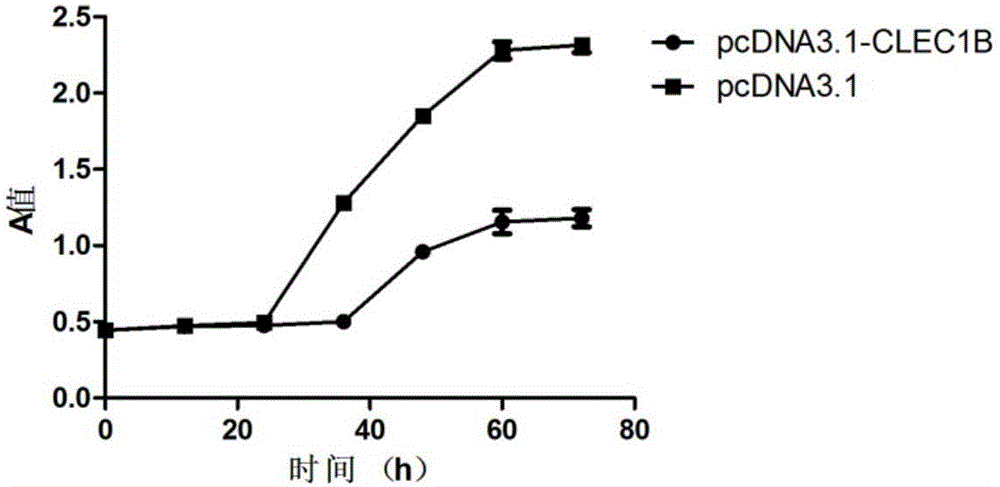
[0093] Example 3 CLEC1B Gene Overexpression
[0094] 1. Human cholangiocarcinoma cell line QBC939 was cultured at 37°C and 5% CO in DMEM (high glucose) medium containing 10% calf serum. 2 , Cultivated in an incubator with a relative humidity of 90%. The medium was changed once every 2-3 days, and 0.25% trypsin was used for routine digestion and passage.
[0095] 2. Overexpression of CLEC1B gene
[0096] 2.1 Construction of CLEC1B gene expression vector
[0097] Amplification primers were designed according to the coding sequence of CLEC1B gene (as shown in SEQIDNO.1), and the primer sequences were as follows: the forward primer was 5'-CCGGGATCCGCCACCATGCAGGATGAAGAT-3' (SEQIDNO.7), and the reverse primer was 5'-CGGCTCGAGAGGTAGTTGGTCCACCTTGG-3 '(SEQ ID NO. 8). The coding sequence of the full-length CLEC1B gene was amplified from the cDNA library of adult fetal brain (clontech company, catalog number: 638831), and the above cDNA sequence was double-digested with restriction e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com