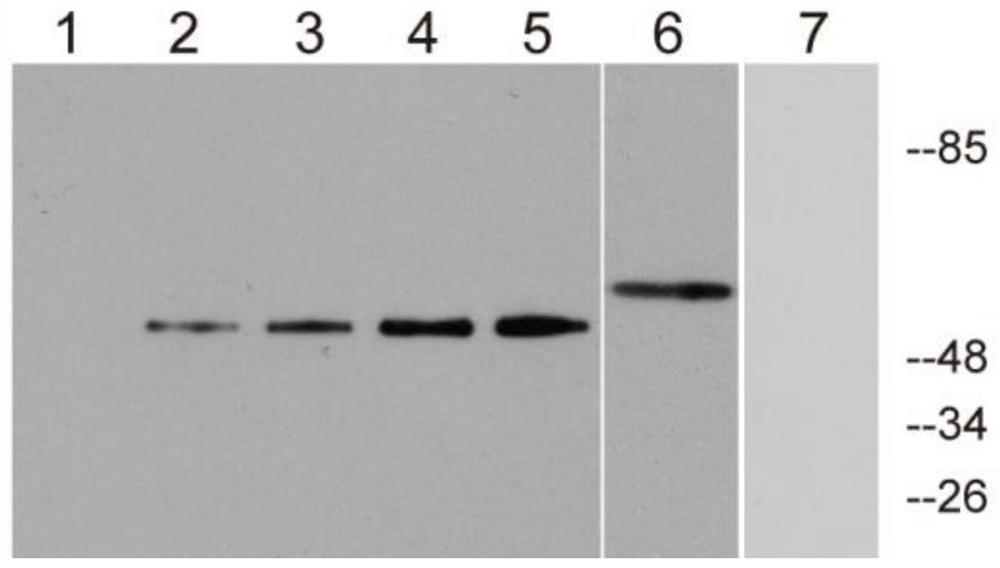
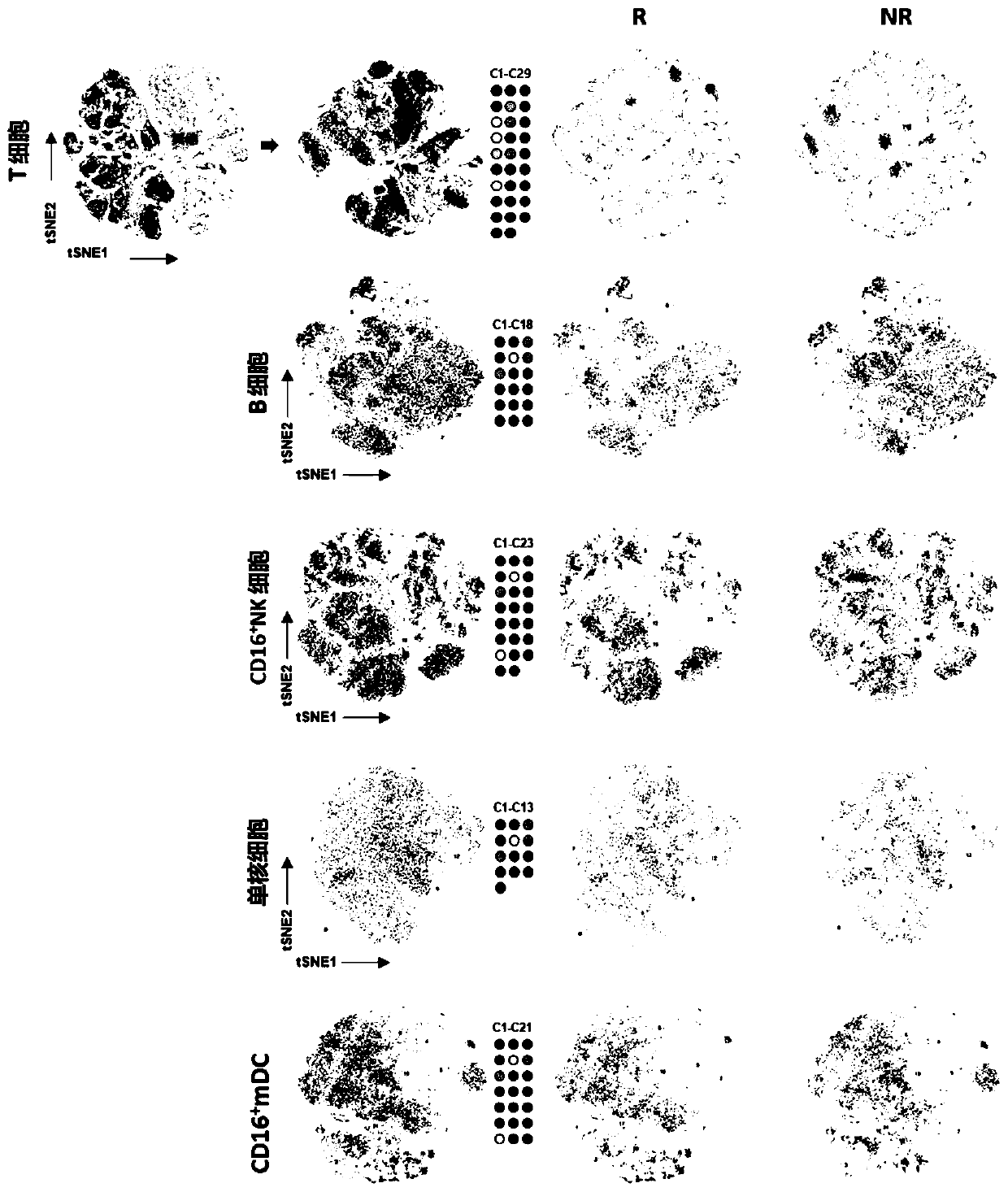
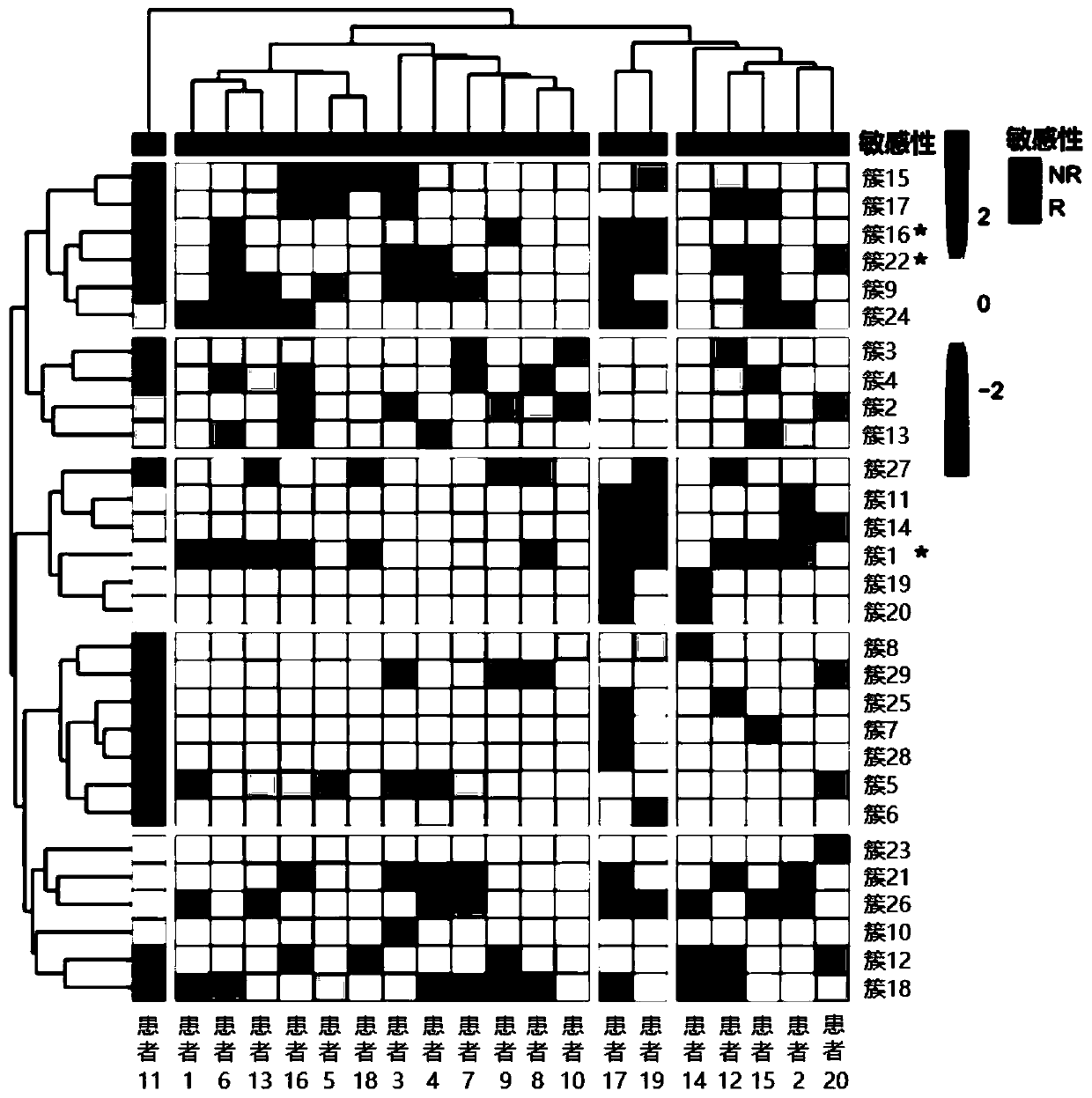
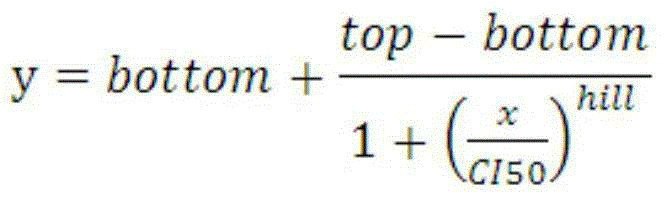Patents
Literature
36 results about "Intrahepatic Cholangiocarcinoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A carcinoma that arises from the intrahepatic bile duct epithelium in any site of the intrahepatic biliary tree. Grossly, the malignant lesions are solid, nodular, and grayish. Morphologically, the vast majority of cases are adenocarcinomas. Signs and symptoms include malaise, weight loss, right upper quadrant abdominal pain, and night sweats. Early detection is difficult and the prognosis is generally poor.
Method For Detecting And Distinguishing Intrahepatic Cholangiocarcinoma
ActiveUS20120065089A1Difficult to differentiateHigh-precision detectionLibrary screeningMaterial analysis by optical meansIntrahepatic CholangiocarcinomaHepatocellular carcinoma
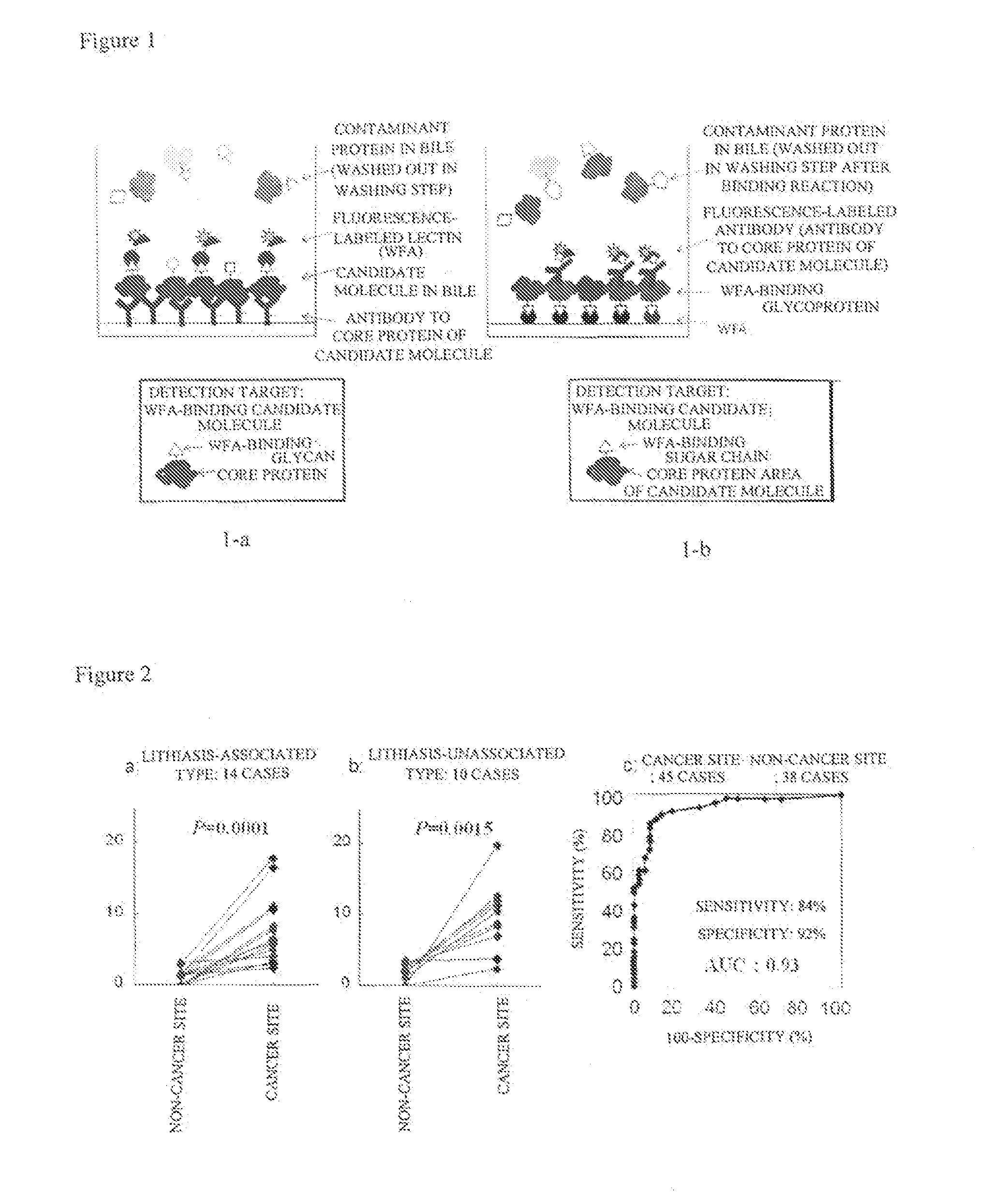
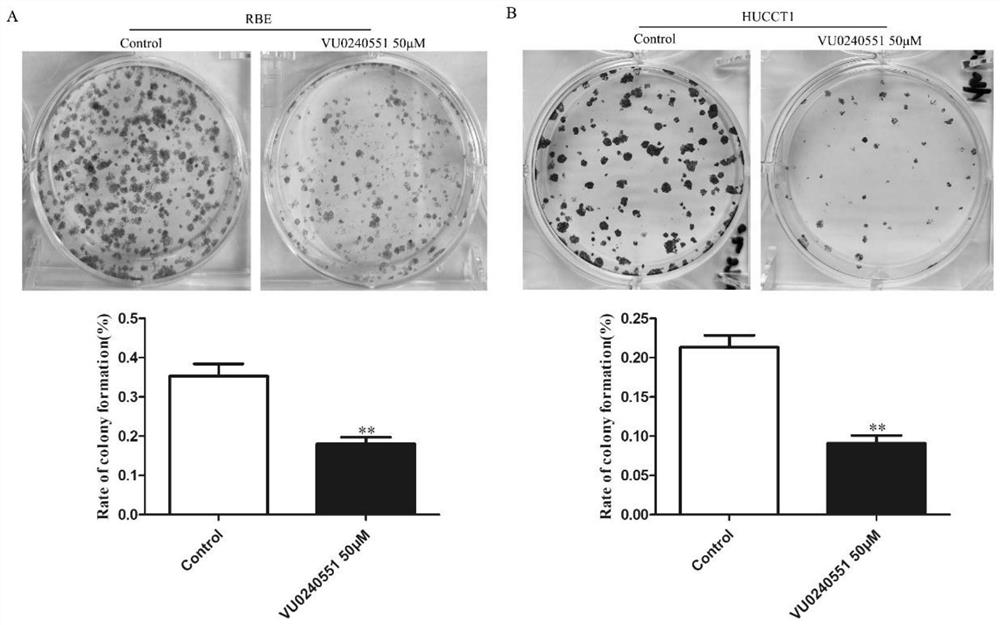
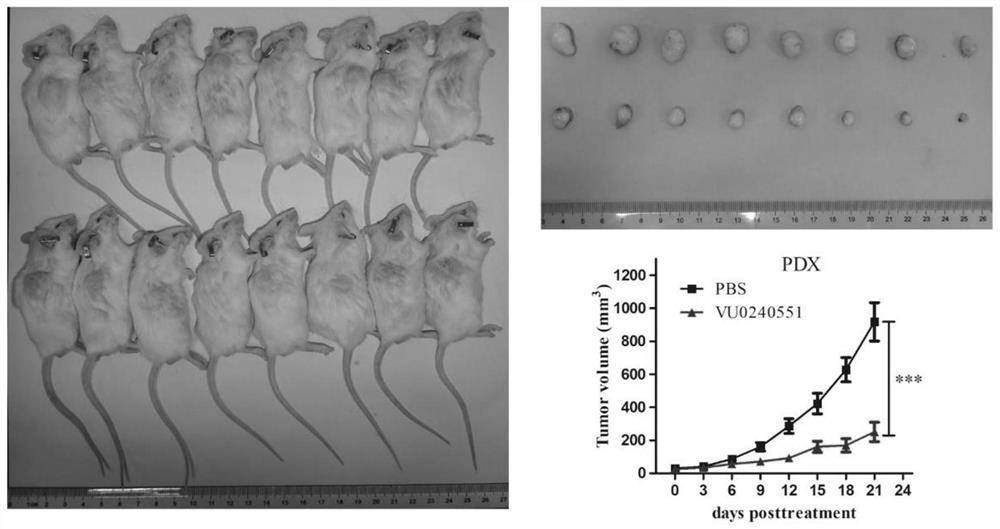
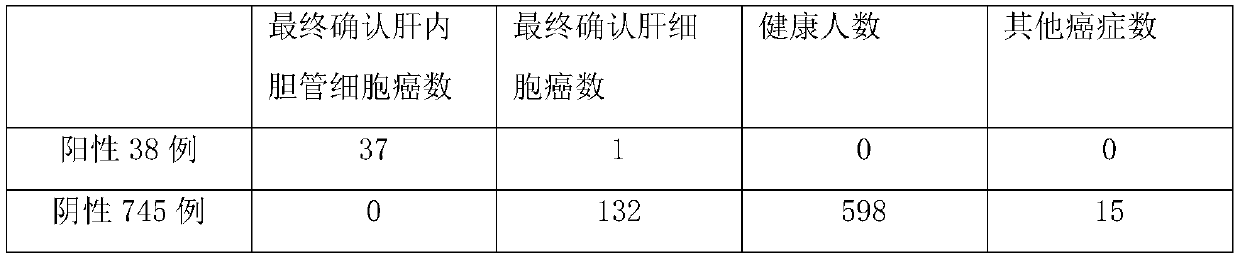
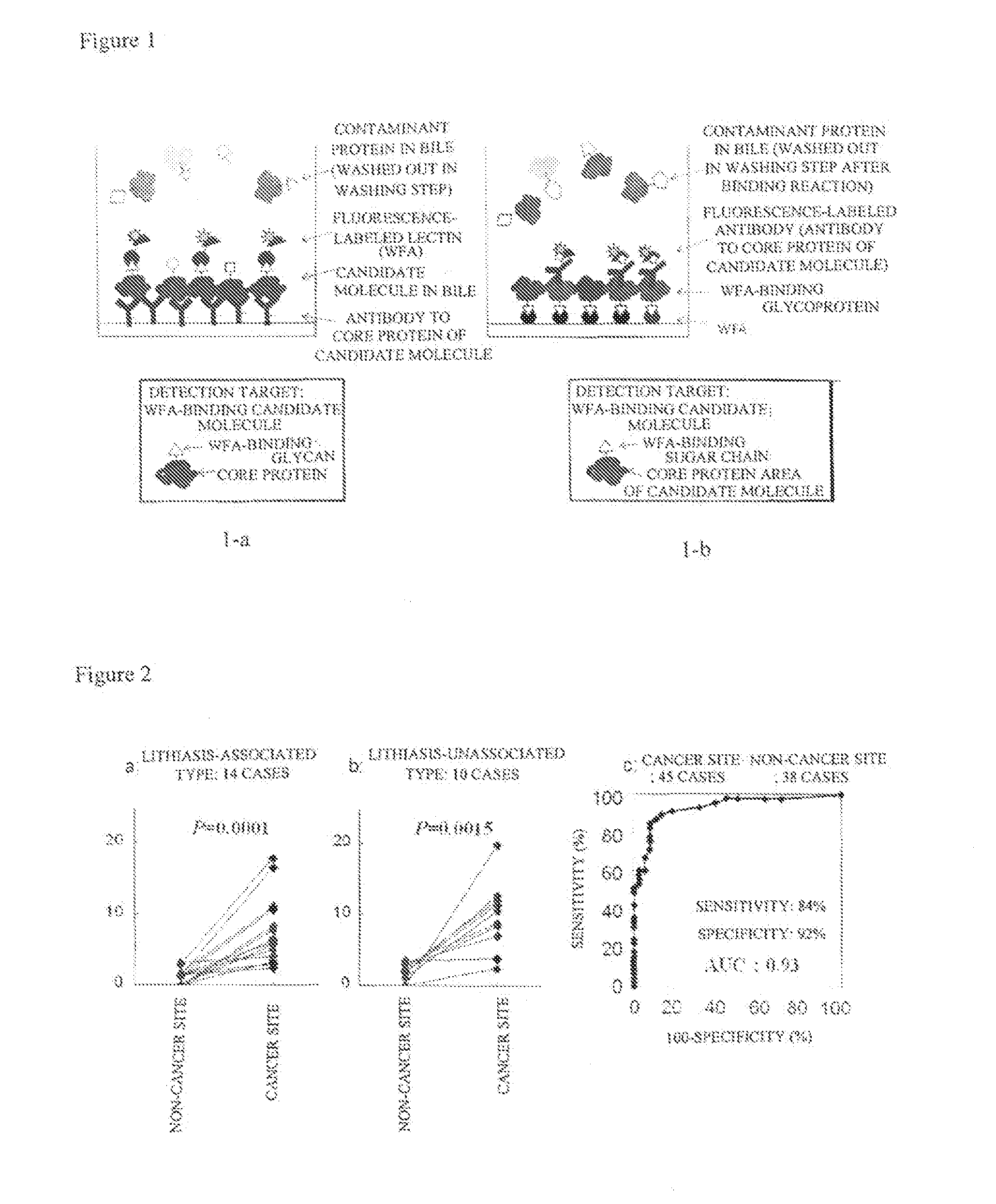
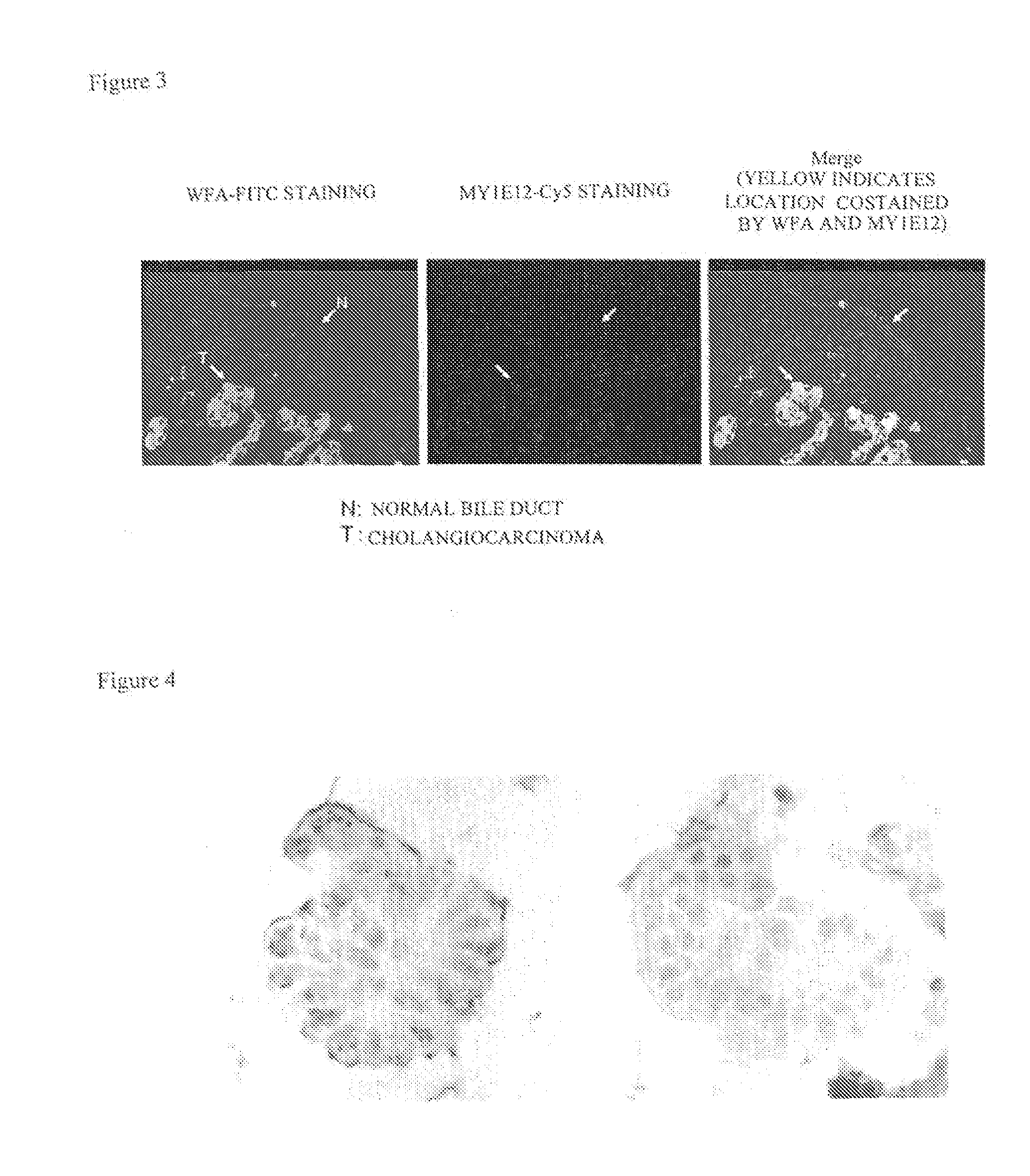
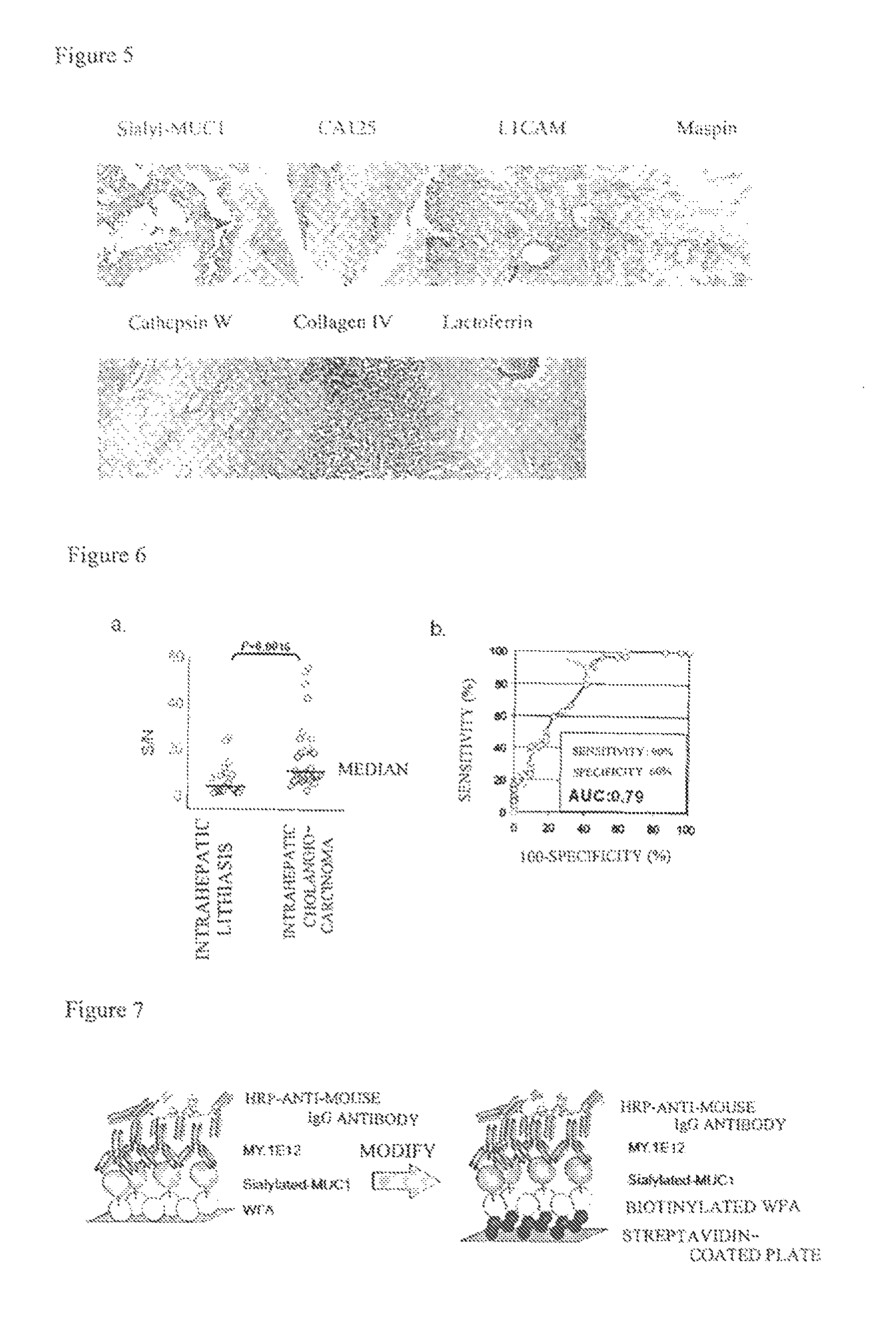
Disclosed are a method for early, sensitively and reliably detecting and distinguishing intrahepatic cholangiocarcinoma in a malignant tumor occurring primarily in the liver in a simple way, and a kit therefor. In the method, a glycan biomarker consisting of a lectin WFA (Wisteria floribunda Agglutinin)-binding glycoprotein derived from intrahepatic cholangiocarcinoma is used as a cancer marker to detect intrahepatic cholangiocarcinoma by detecting the cancer marker in a test specimen. The method for detecting intrahepatic cholangiocarcinoma can clearly differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma and enables early detection and determination with a performance clinically acceptable in terms of applicability, sensitivity and precision.
Owner:NAT INST OF ADVANCED IND SCI & TECH +1
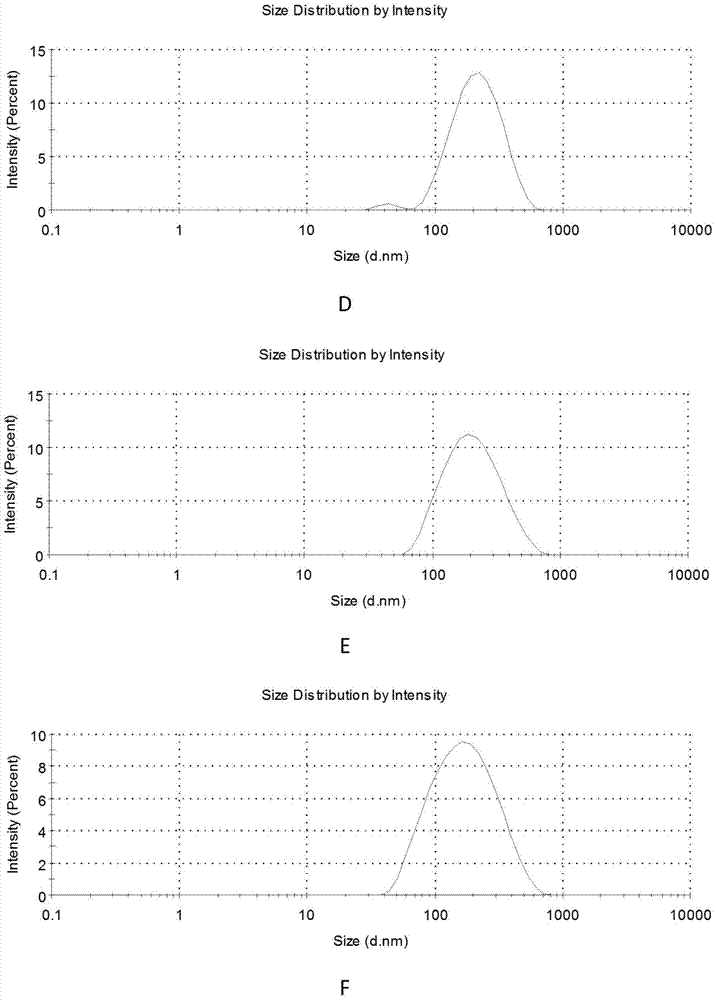
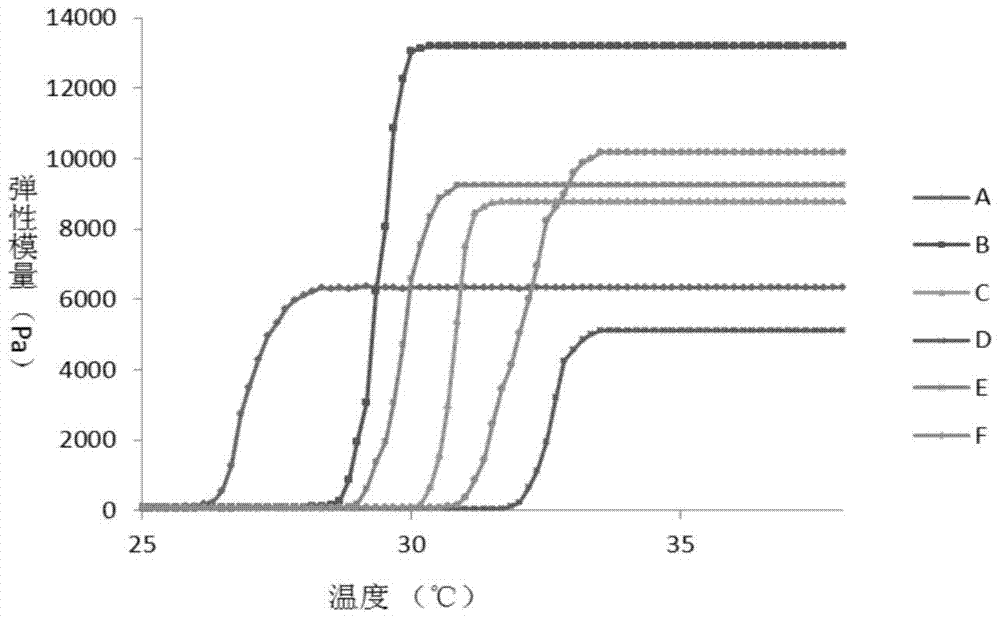
Pharmaceutical nanometer composite temperature-sensitive gel for treating biliary tract tumor
ActiveCN106994117AWith shippingWith tumor treatmentHeavy metal active ingredientsAerosol deliveryIntrahepatic CholangiocarcinomaBiliary tract
The invention relates to a pharmaceutical nanometer composite temperature-sensitive gel for treating a biliary tract tumor. The gel is made by coating nanometer particles made from an anti-tumor active substance with a polymer or a targeted-gene-modified polymer which is taken as a carrier material, and adding a temperature-sensitive polymer material to prepare nanometer composite temperature-sensitive gel, or dissolving or suspending the anti-tumor active substance nanometer particles in temperature-sensitive gel. The gel is simple to prepare, is suitable for massive production, and is suitable for preparing a medicine for treating and preventing biliary tract tumors, such as gallbladder carcinoma, intrahepatic cholangiocarcinoma and cholangiocarcinoma, which relapse after operation.
Owner:上海佶珥技术服务中心 +1
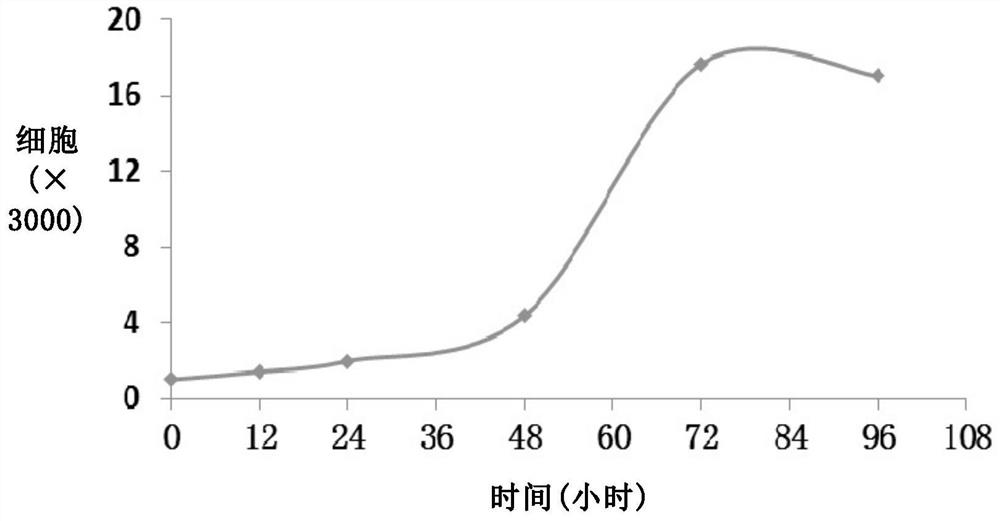
Human intrahepatic duct cancer cell line and application thereof
InactiveCN104560878AAlgebraically clearNo cross contaminationMicrobiological testing/measurementMicroorganism based processesIntrahepatic CholangiocarcinomaIn vivo
The invention relates to a human intrahepatic duct cancer cell line and an application thereof. The human intrahepatic duct cancer cell line LICCF is established according to the invention, is clear and definite in algebra, does not have cross contamination, shows unique sensitivity to an inhibitor of a PI3K / mTOR passage and an inhibitor of an MEK1 / 2 passage, has high drug resistance to 5-FU, cis-platinum and FGFR inhibitors, and can be used for establishing in vivo and in vitro tumor drug experiment and drug resistance models, so that an experimental basis is provided for revealing a human intrahepatic duct cancer drug resistance mechanism, reversing the appearance of drug resistance, developing new anticancer drugs and screening human intrahepatic duct cancer related biological markers.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
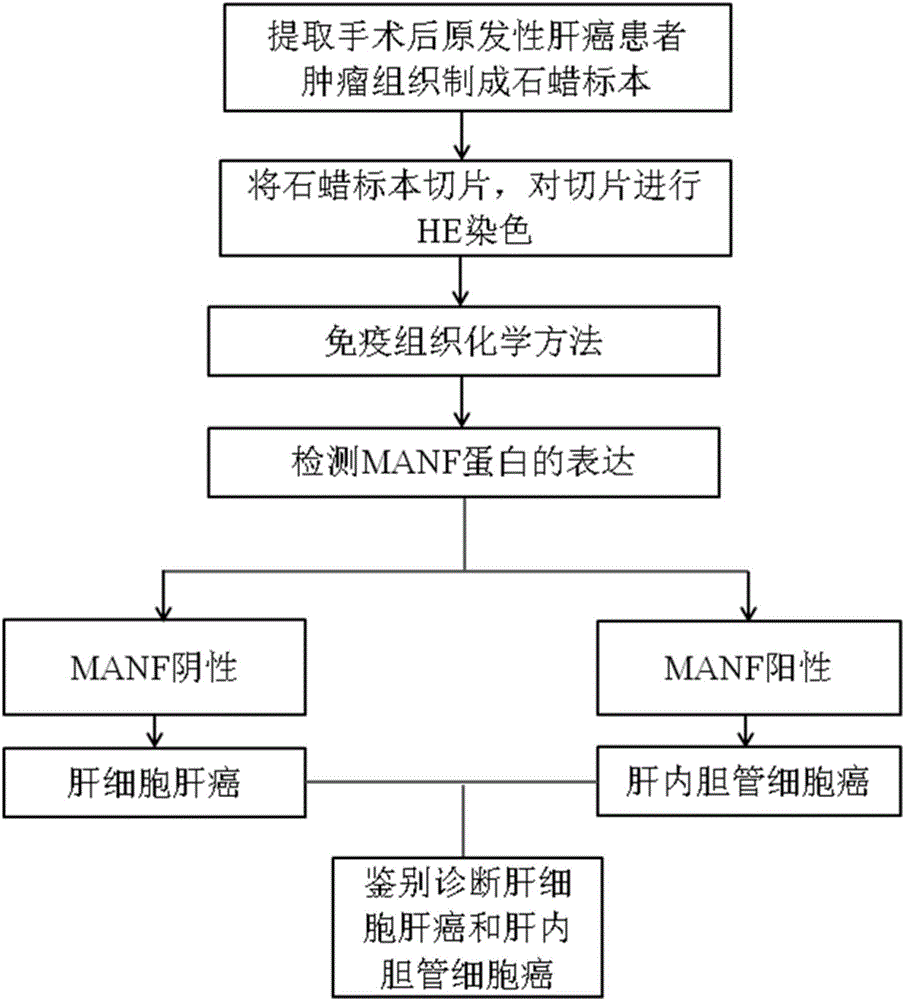
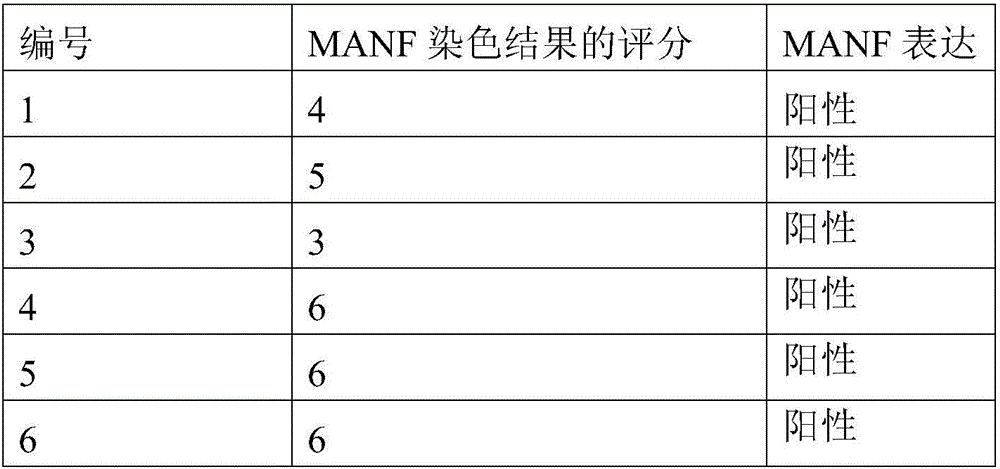
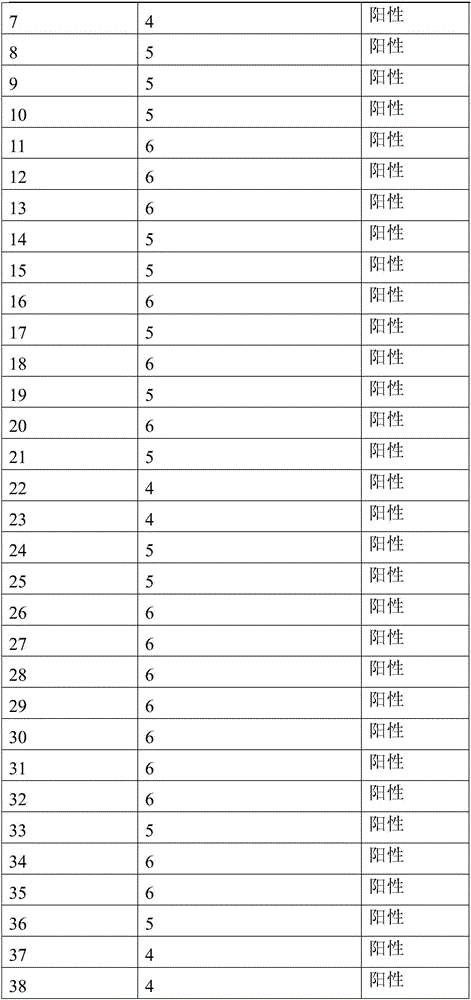
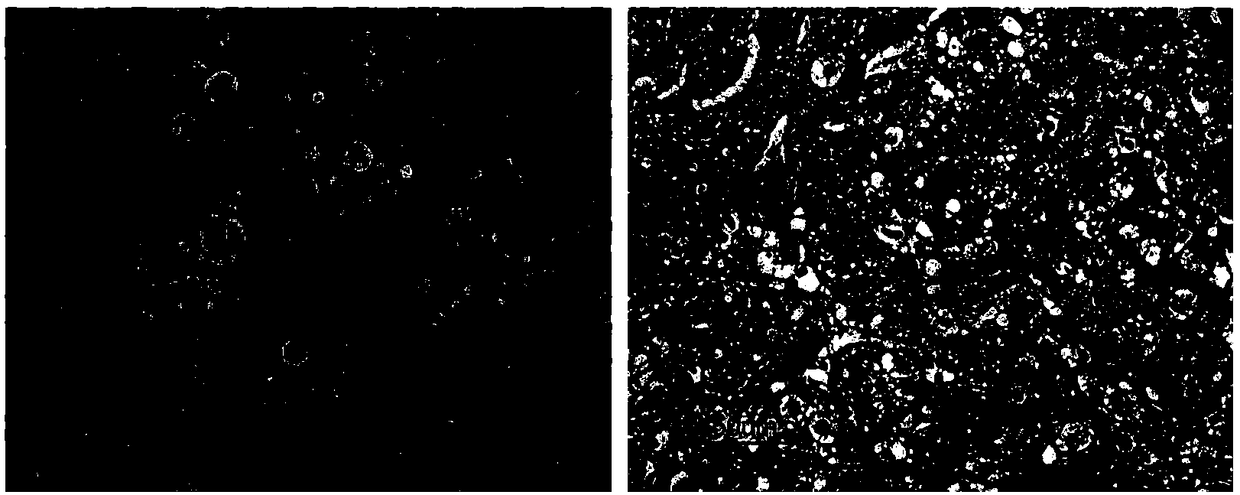
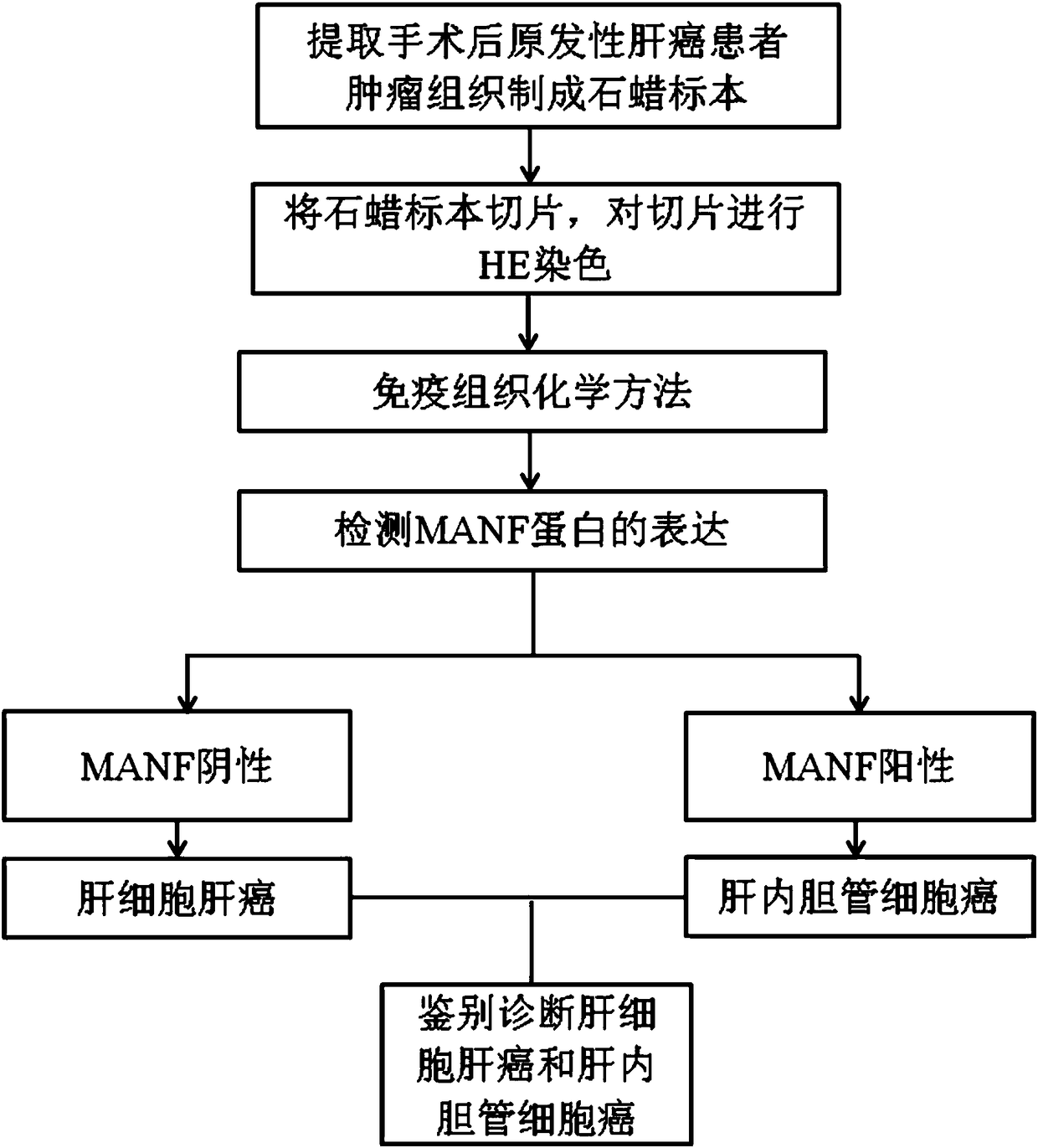
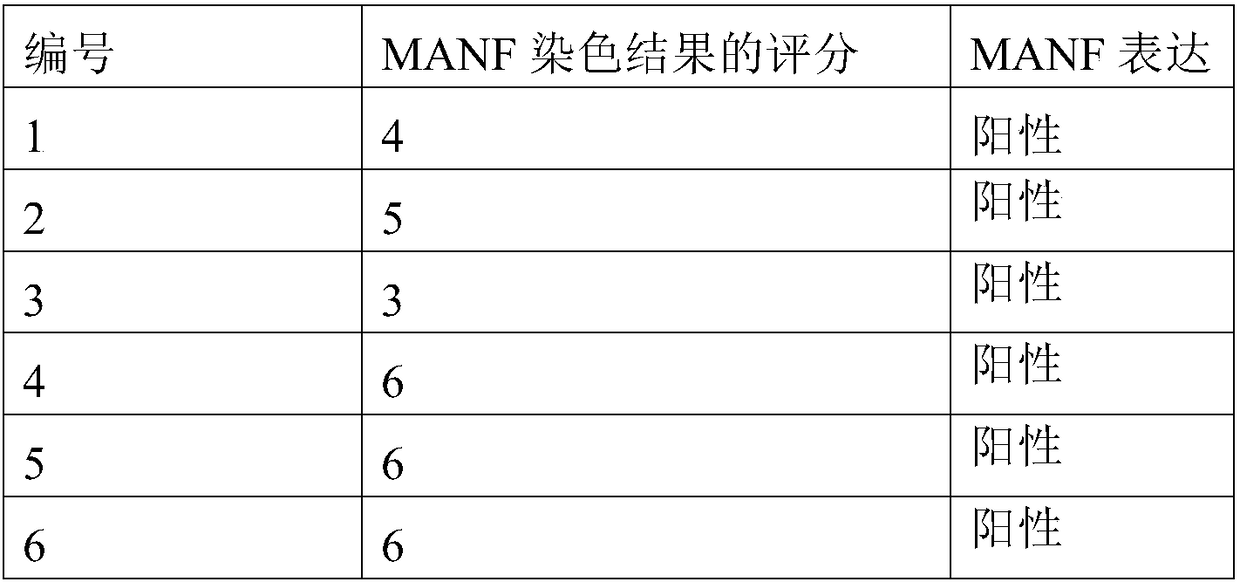
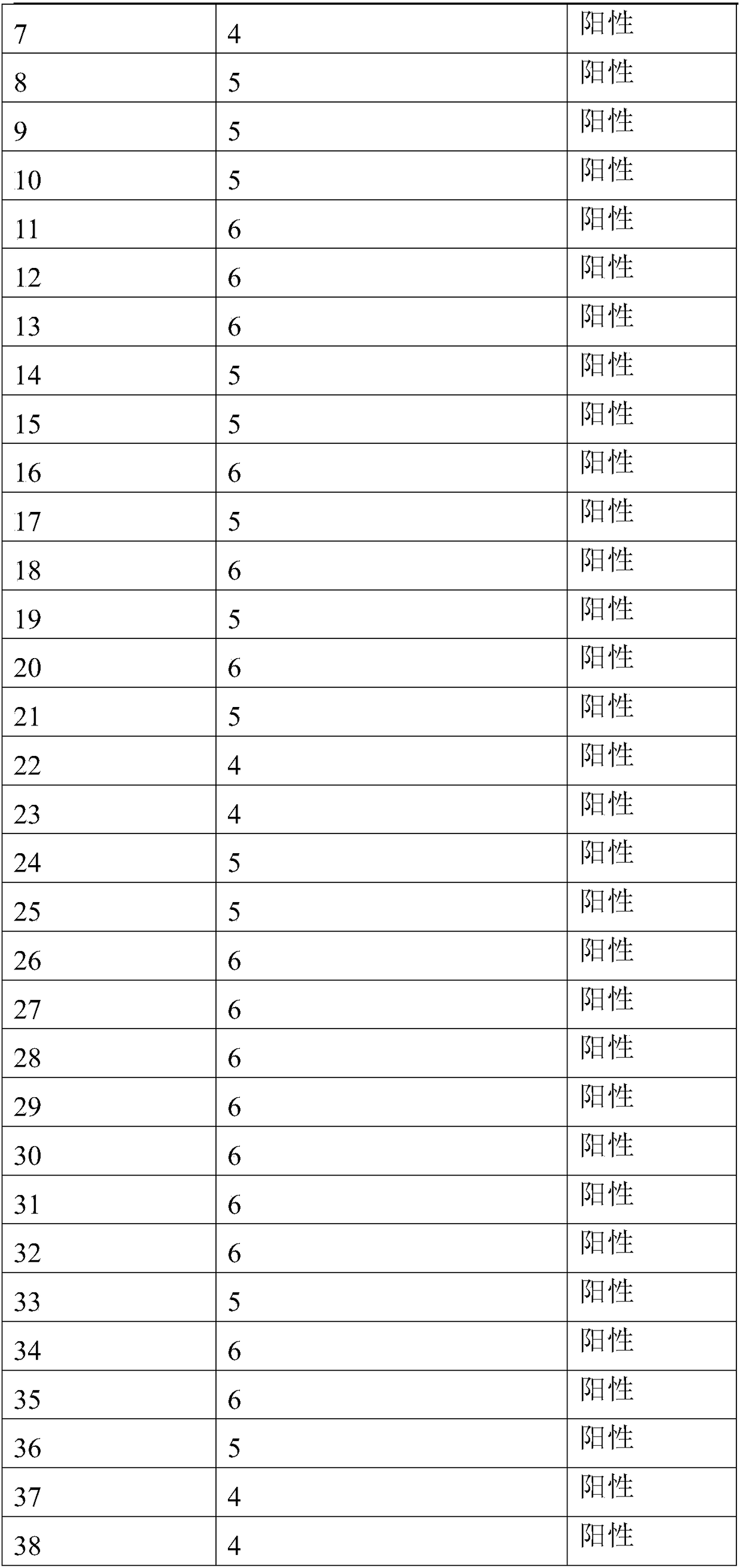
Product and method for identifying hepatocellular carcinoma and intrahepatic cholangiocarcinoma based on MANF as marker
ActiveCN106645725AImproved prognosisImprove the quality of lifeMaterial analysisIntrahepatic CholangiocarcinomaIntrahepatic bile ducts
The invention discloses a product and a method for identifying hepatocellular carcinoma and intrahepatic cholangiocarcinoma based on an MANF as a marker. The invention provides an application of a system for detecting the expression quantity of the MANF in preparation of products for identification or supplementary identification of hepatocellular carcinoma patients and intrahepatic cholangiocarcinoma patients. The hepatocellular carcinoma and the intrahepatic cholangiocarcinoma are identified by taking the MANF as the marker and detecting the expression level of the MANF in a primary hepatocellular carcinoma tissue, a method and an index for effectively identifying the hepatocellular carcinoma and the intrahepatic cholangiocarcinoma are built, the sensitivity is 92.5% and the specificity is 74.7%. A foundation is laid for targeted appropriate treatment and improvement of prognosis of the patients to improve the living quality of the patients and to reduce the economic burden of the patients.
Owner:ANHUI MEDICAL UNIV
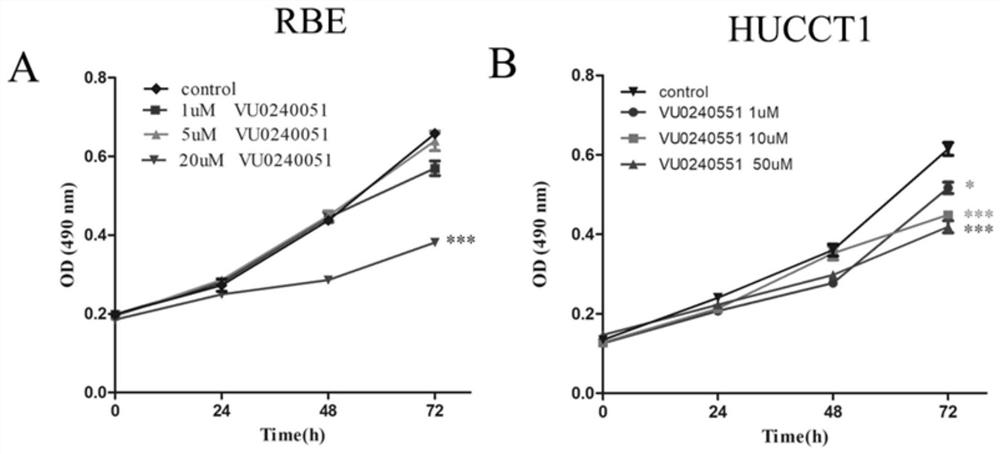
Application of substance taking MANF as target spot in preparing products for treating intrahepatic cholangiocarcinoma
ActiveCN109364249APrevent proliferationInhibit migrationOrganic active ingredientsDigestive systemIntrahepatic CholangiocarcinomaMedicine
The invention discloses an application of a substance taking MANF as a target spot in preparing products for treating intrahepatic cholangiocarcinoma. An experiment proves that the substance for inhibiting MANF expression in cells or inhibiting MANF activity in cells is capable of effectively inhibiting the multiplication, migration and / or invasion of cholangiocarcinoma cells, is capable of inhibiting the growth of cholangiocarcinoma and can be used for treating or / and preventing intrahepatic cholangiocarcinoma.
Owner:ANHUI MEDICAL UNIV
Human intrahepatic cholangiocarcinoma cell line with high tumorigenic ability and application thereof
InactiveCN107177551AHigh tumorigenic abilityMicrobiological testing/measurementMicroorganism based processesIntrahepatic CholangiocarcinomaMalignant progression
The invention relates to a human intrahepatic cholangiocarcinoma cell line with high tumorigenic ability and application thereof. The invention discloses a novel human intrahepatic cholangiocarcinoma cell line which grows stably and has clear quantity of generations and high tumorigenic rate; the human intrahepatic cholangiocarcinoma cell line can form an adenoid tumor in vivo and can be used for in-vivo and in-vitro research on human intrahepatic cholangiocarcinoma; a foundation is laid for constructing an in-vivo animal model, disclosing a tumor malignant progression mechanism, screening related biomarkers and developing novel intrahepatic cholangiocarcinoma resisting medicines.
Owner:HEPATOBILIARY SURGERY HOSPITAL SECOND MILITARY MEDICAL UNIV
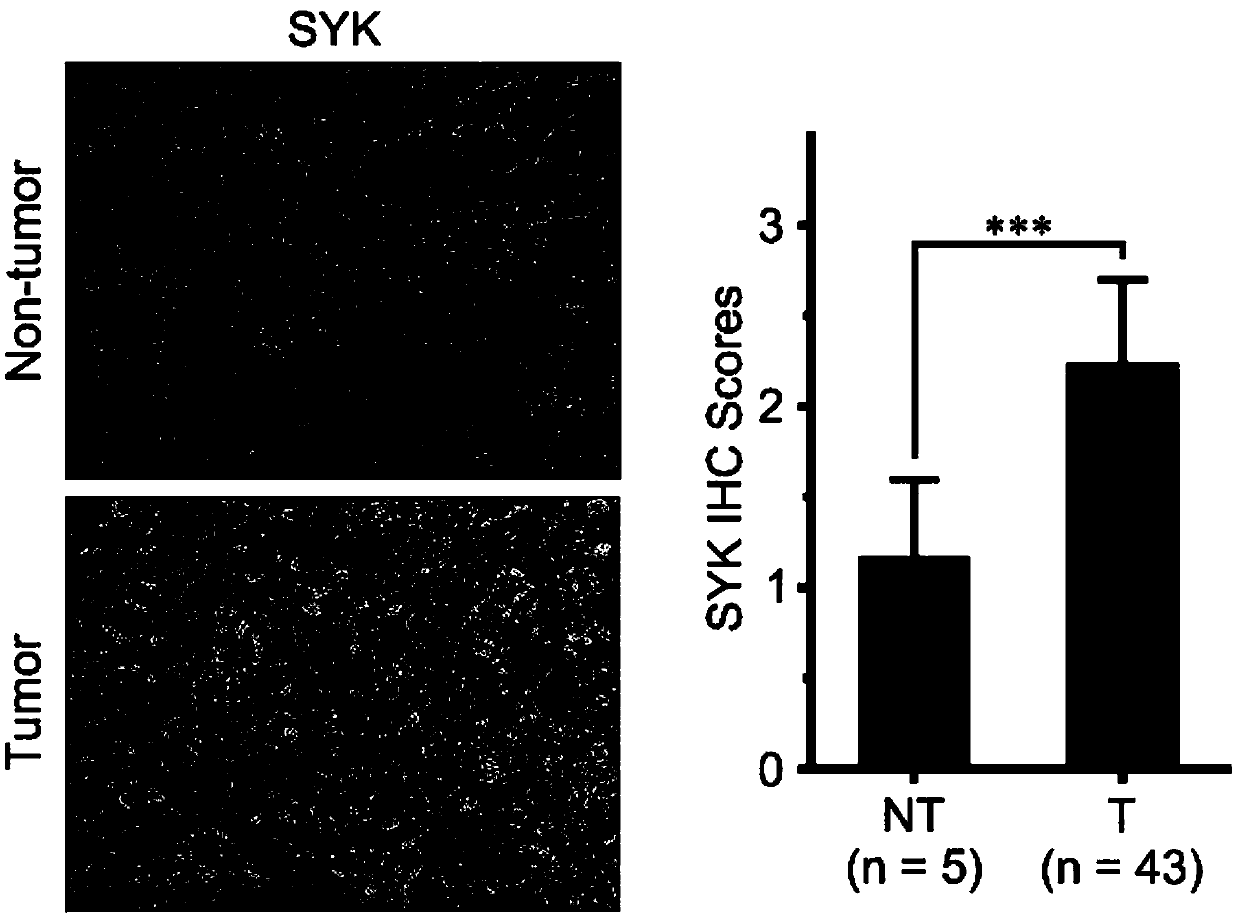
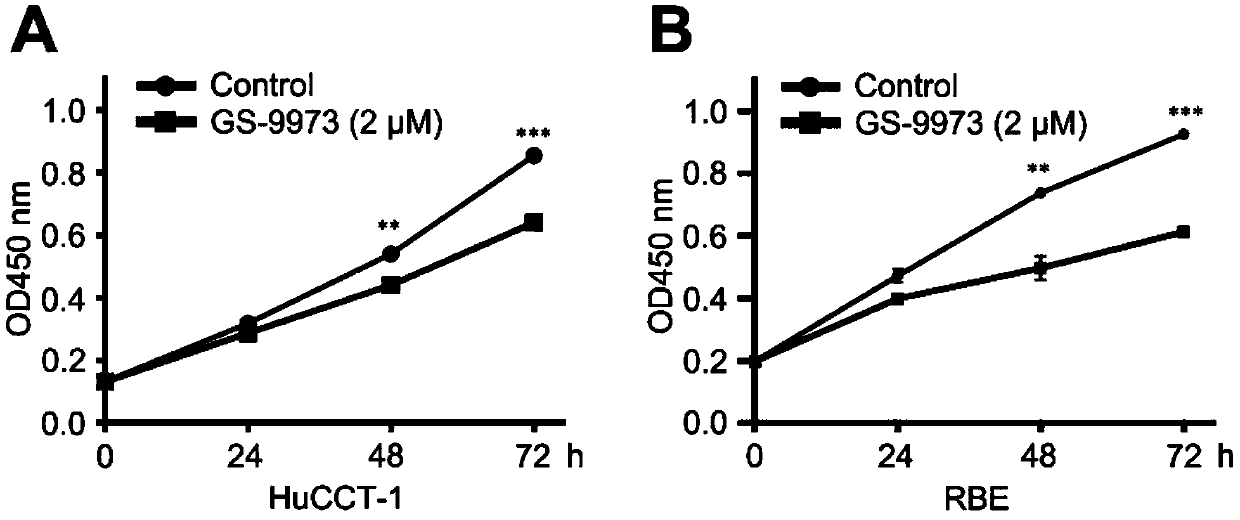
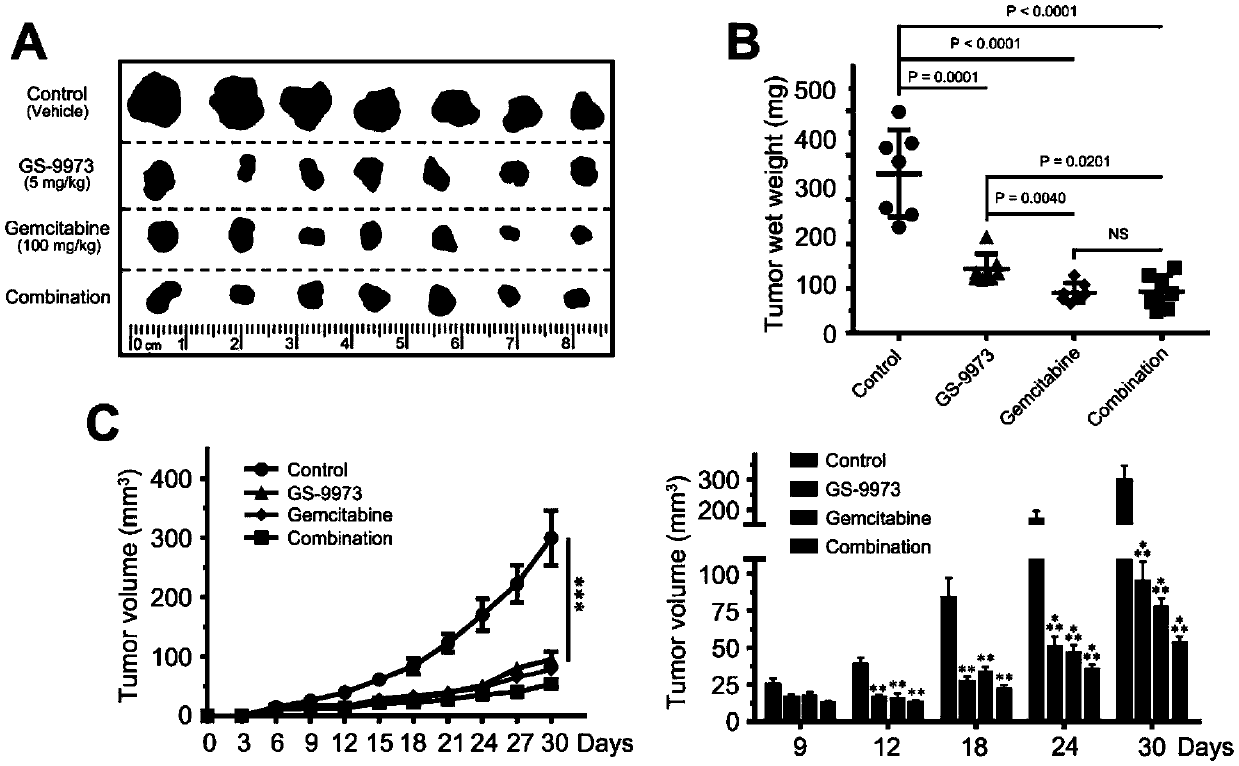
Application of splenic tyrosine kinase as therapeutic target for intrahepatic cholangiocarcinoma
ActiveCN109529041APrevent proliferationOrganic active ingredientsAntineoplastic agentsIntrahepatic CholangiocarcinomaChemotherapeutic drugs
The invention discloses an application of splenic tyrosine kinase as a therapeutic target for intrahepatic cholangiocarcinoma. Experiments find out that the expression of SYK in intrahepatic cholangiocarcinoma is increased. In-vitro cell and in-vivo animal experiments find out that the proliferation of intrahepatic cholangiocarcinoma can be effectively inhibited with the use of the SYK antagonistGS-9973, and the inhibiting effect is close to that of a first-line chemotherapeutic drug gemcitabine.
Owner:TCM INTEGRATED HOSPITAL OF SOUTHERN MEDICAL UNIV
A human intrahepatic cholangiocarcinoma cell line with high tumorigenic ability and its application
InactiveCN107177551BHigh tumorigenic abilityMicrobiological testing/measurementMicroorganism based processesIntrahepatic CholangiocarcinomaBiomarker (medicine)
The invention relates to a human intrahepatic cholangiocarcinoma cell line with high tumorigenic ability and application thereof. The invention discloses a novel human intrahepatic cholangiocarcinoma cell line which grows stably and has clear quantity of generations and high tumorigenic rate; the human intrahepatic cholangiocarcinoma cell line can form an adenoid tumor in vivo and can be used for in-vivo and in-vitro research on human intrahepatic cholangiocarcinoma; a foundation is laid for constructing an in-vivo animal model, disclosing a tumor malignant progression mechanism, screening related biomarkers and developing novel intrahepatic cholangiocarcinoma resisting medicines.
Owner:HEPATOBILIARY SURGERY HOSPITAL SECOND MILITARY MEDICAL UNIV
Prognosis detection biomarker and detection kit for intrahepatic cholangiocarcinoma patient
ActiveCN112280868ARealize non-invasive testingWith non-invasive detectionMicrobiological testing/measurementDNA/RNA fragmentationIntrahepatic CholangiocarcinomaBiologic marker
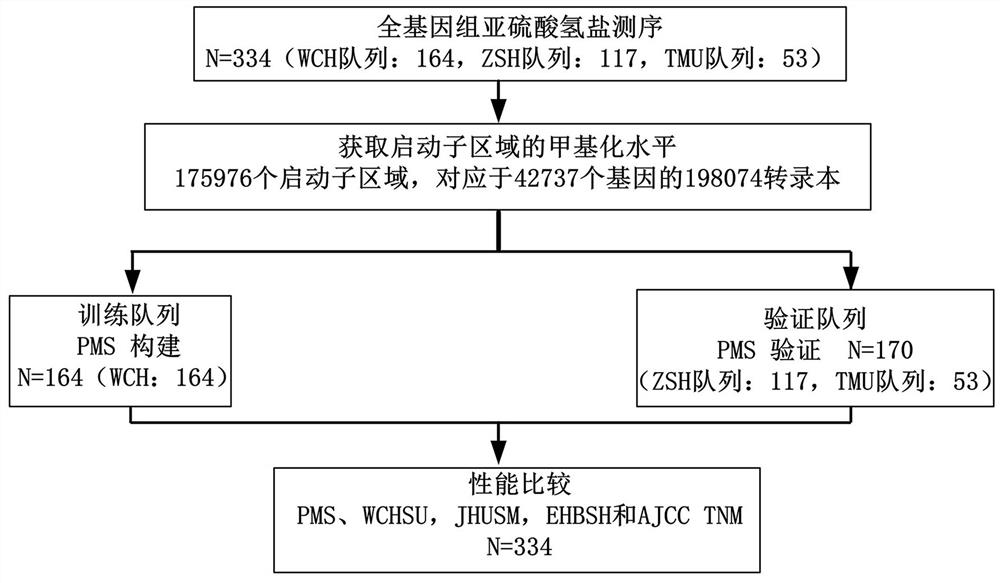
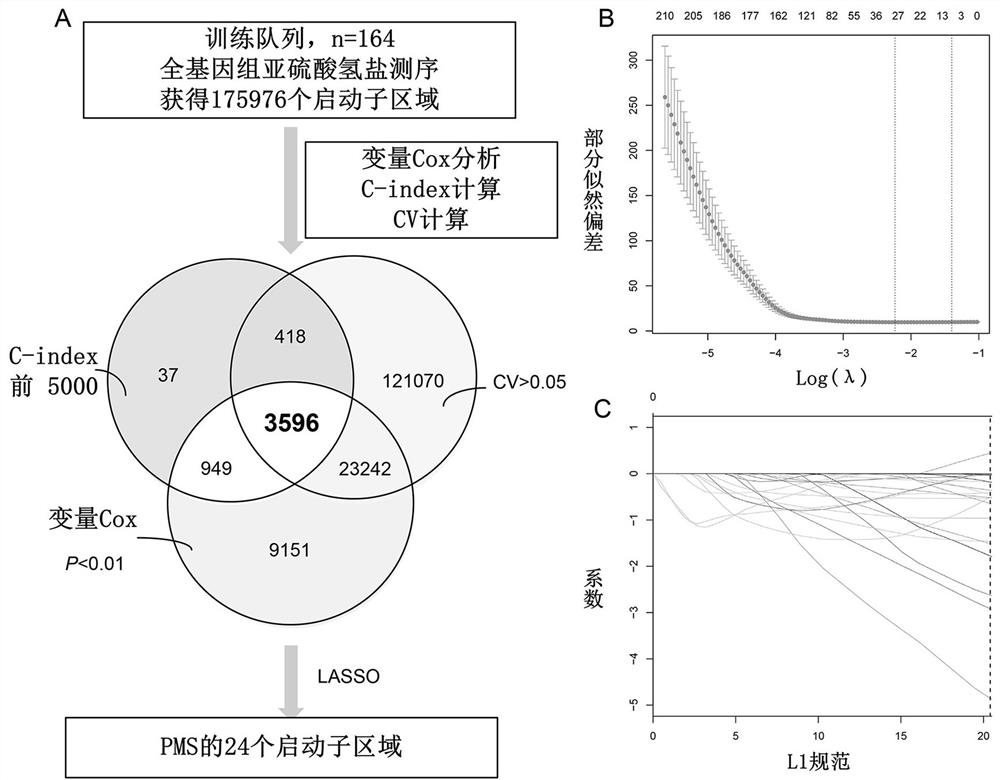
The invention discloses a prognosis detection biomarker and a detection kit for an intrahepatic cholangiocarcinoma patient. A set of polynucleotide sequences shown as SEQ ID NO.1 -SEQ ID NO.24 is taken as a biomarker, methylation analysis is performed on promoter regions, an effective methylation scoring model is constructed, and accurate evaluation of prognosis lifetime of the intrahepatic cholangiocarcinoma patient is realized. The biomarker can be used as a single prediction factor for prognosis of the patient suffering from the intrahepatic cholangiocarcinoma, and can also be used for predicting the prognosis lifetime of the patient suffering from the intrahepatic cholangiocarcinoma based on a plurality of variables including methylation scores of gene promoters.
Owner:浙江高美生物科技有限公司
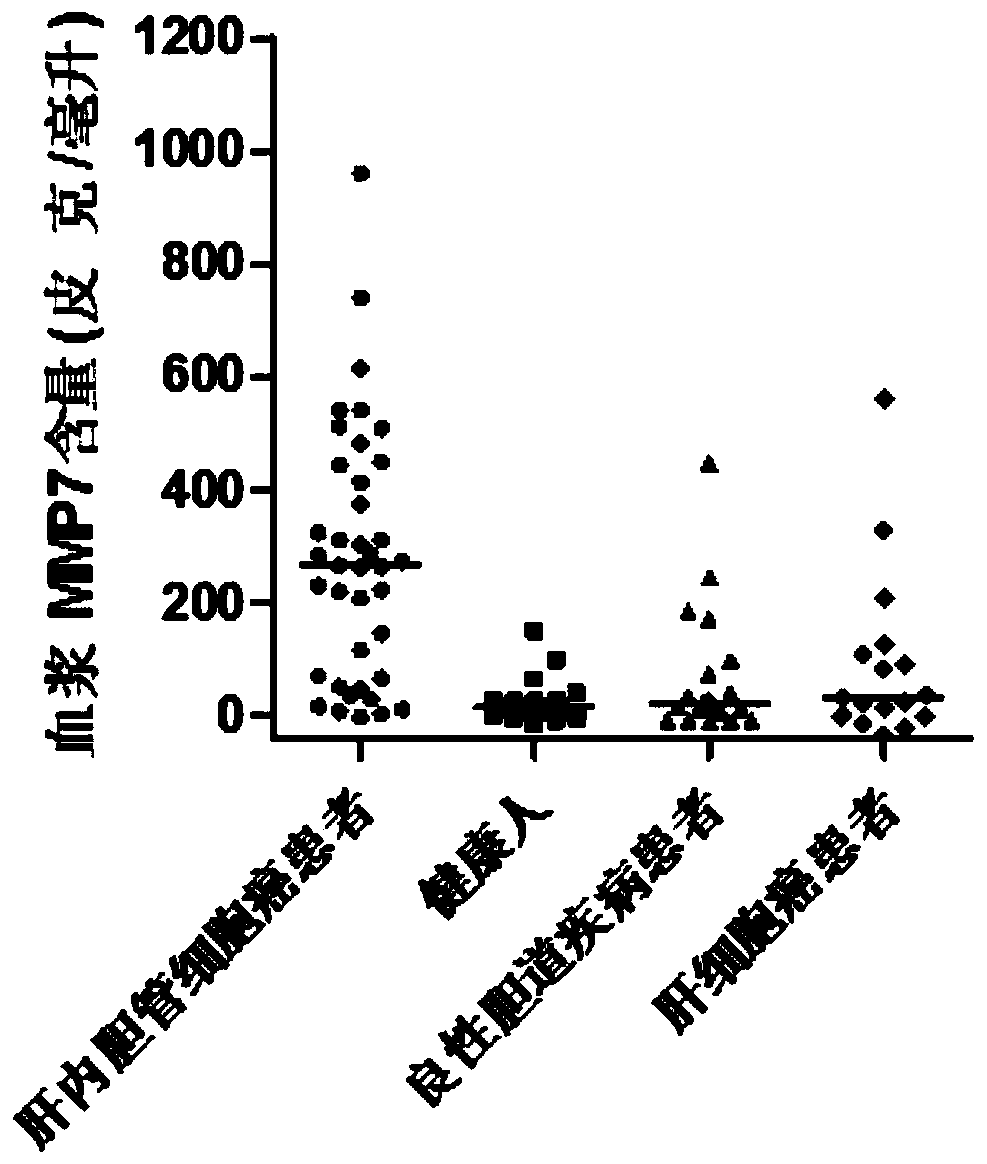
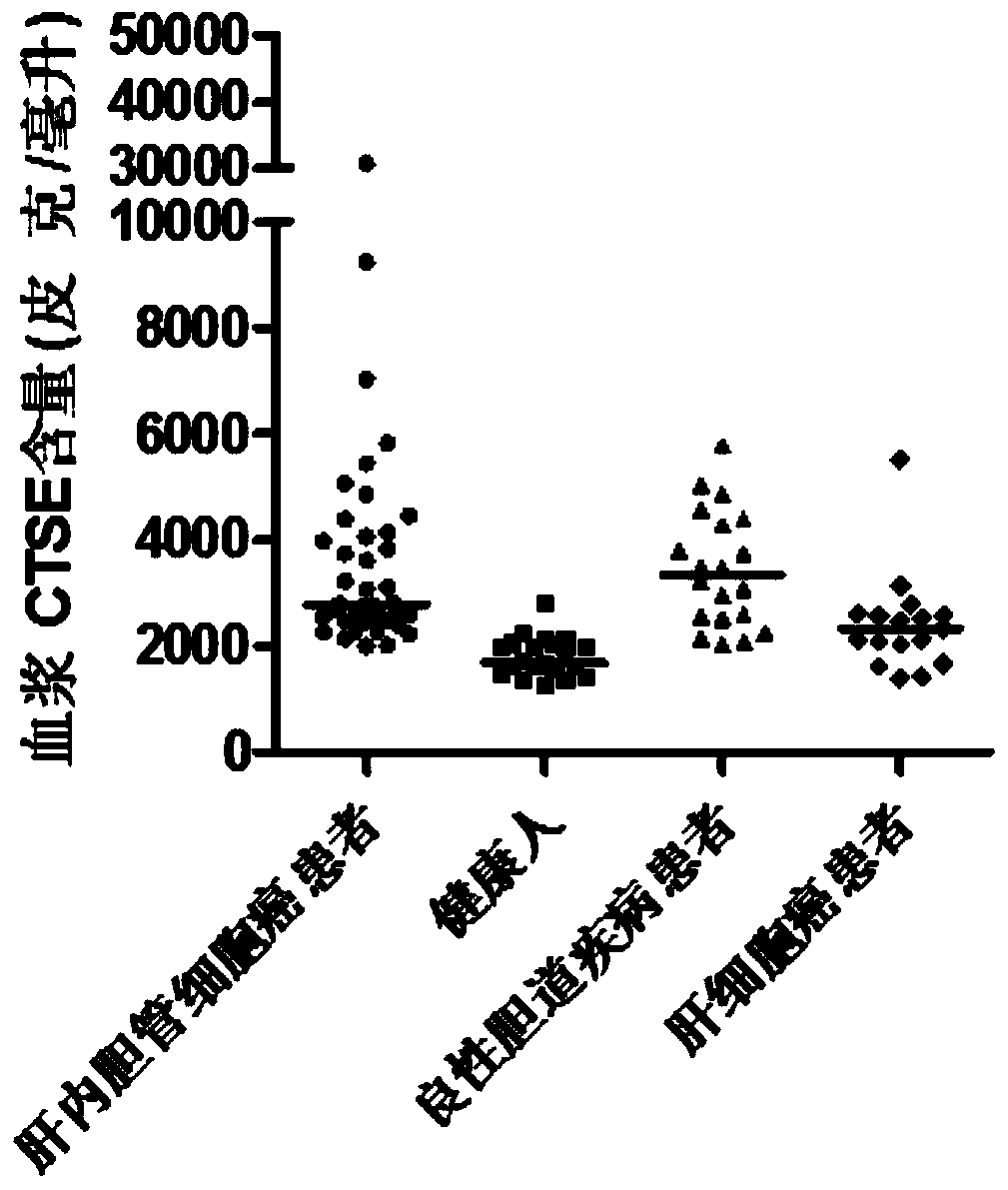
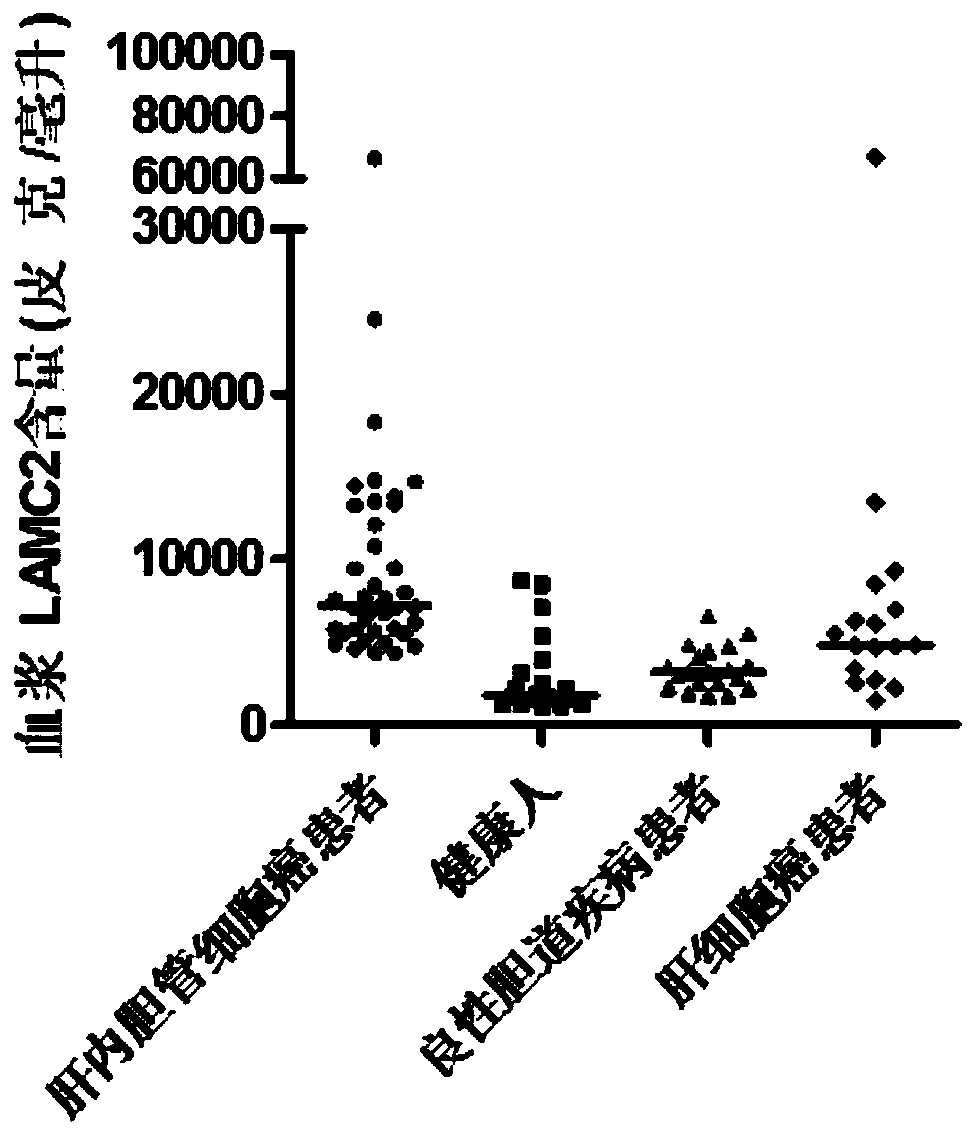
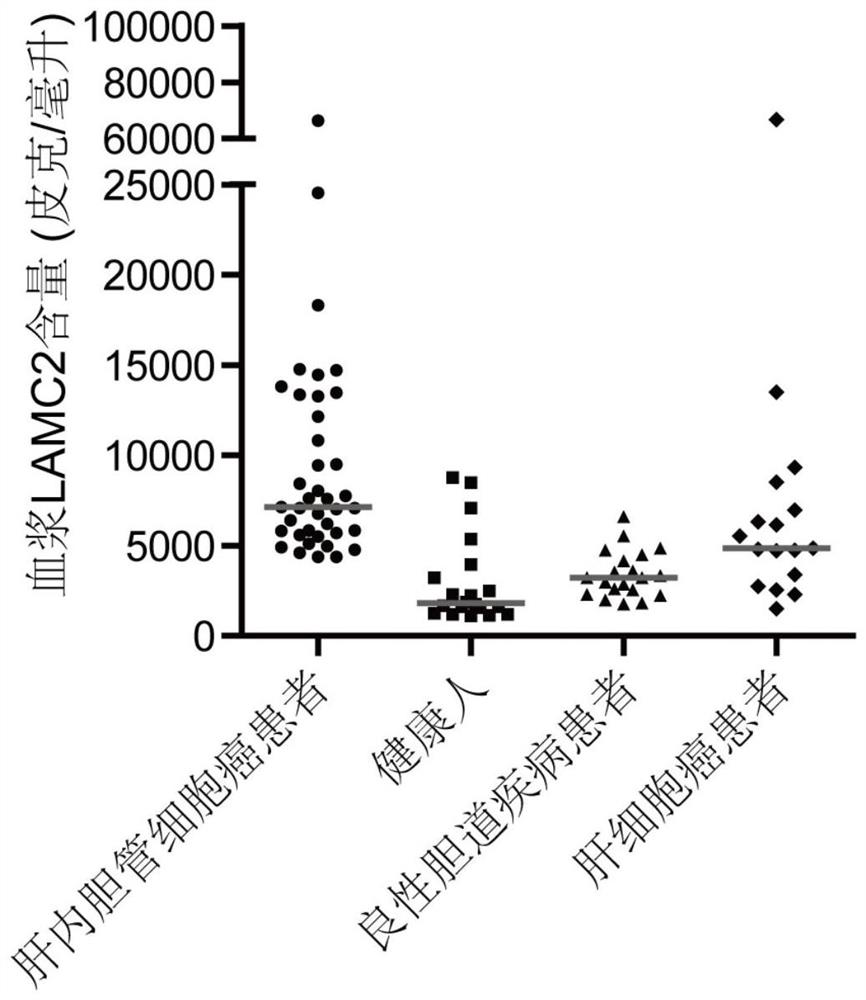
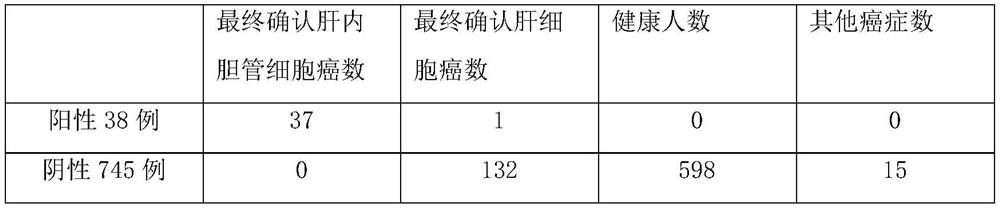
Application of MMP7, CTSE or LAMC2 protein in preparation of diagnosis reagent for intrahepatic cholangiocarcinoma
InactiveCN110736840ABiological material analysisBiological testingDiseaseIntrahepatic Cholangiocarcinoma
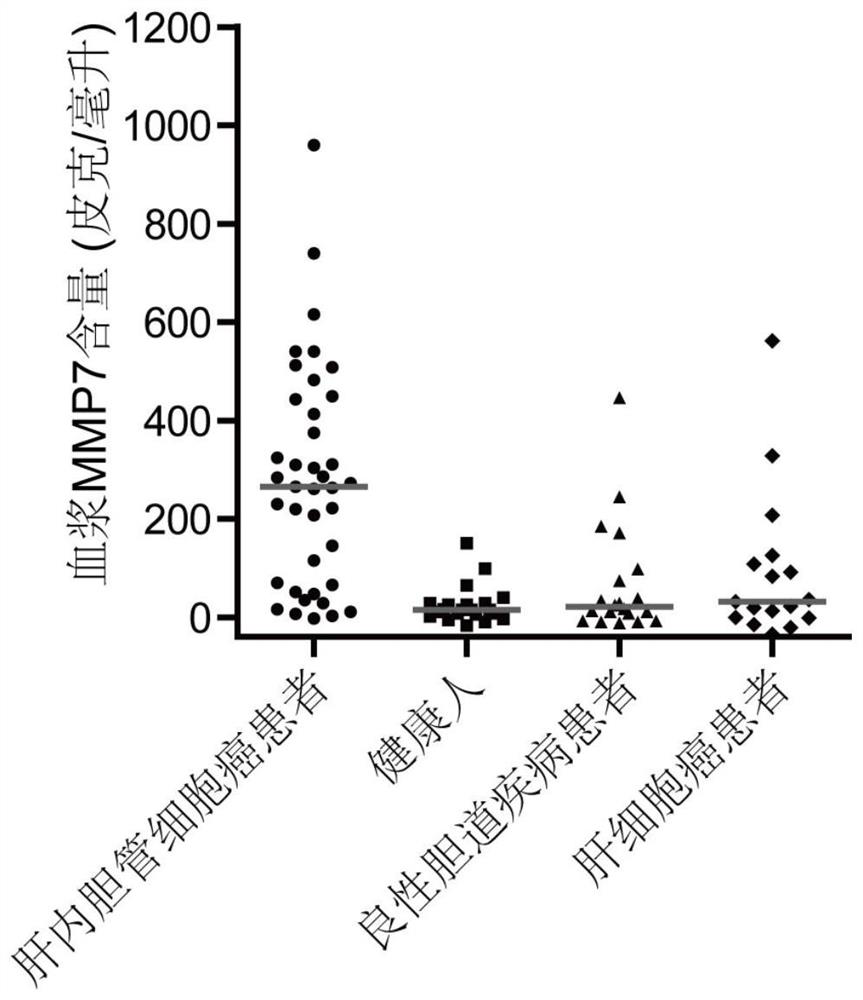
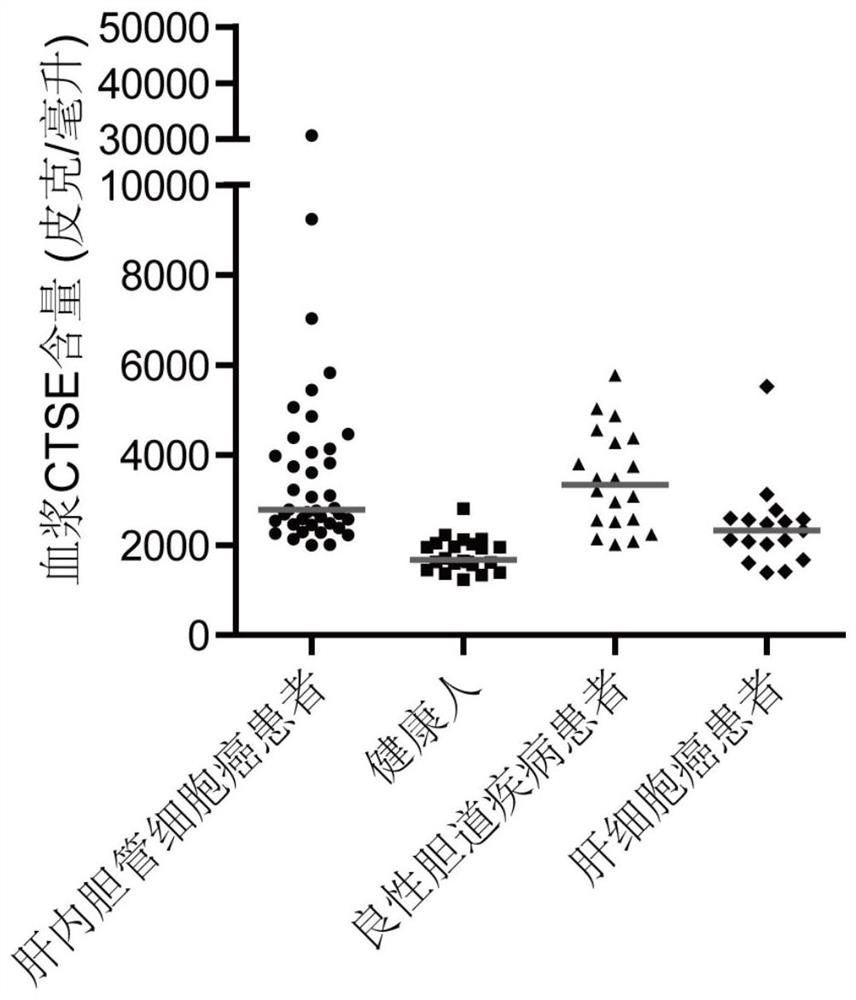
The invention relates to the technical field of medical bioengineering, and aims to provide application of MMP7, CTSE or LAMC2 protein in preparation of a diagnosis reagent for intrahepatic cholangiocarcinoma. At least one protein is used for preparing a reagent capable of identifying and diagnosing intrahepatic cholangiocarcinoma and distinguishing primary hepatocellular carcinoma, namely, the three proteins are used independently or in combination to identify and distinguish hepatocellular carcinoma and intrahepatic cholangiocarcinoma. The invention provides a reagent or kit product preparedfrom the three proteins, and the expression level of the protein in blood is detected by adopting an immunological related method to serve as a basis for diagnosing an intrahepatic cholangiocarcinomamarker. The possibility of judging intrahepatic cholangiocarcinoma, benign biliary tract diseases and hepatocellular carcinoma for a subject, and the possibility of judging the health state for the subject can be accurately and specifically provided for the subject, and a basis is provided for a next diagnosis and treatment scheme of a doctor.
Owner:杭州道合天孚生物科技有限公司
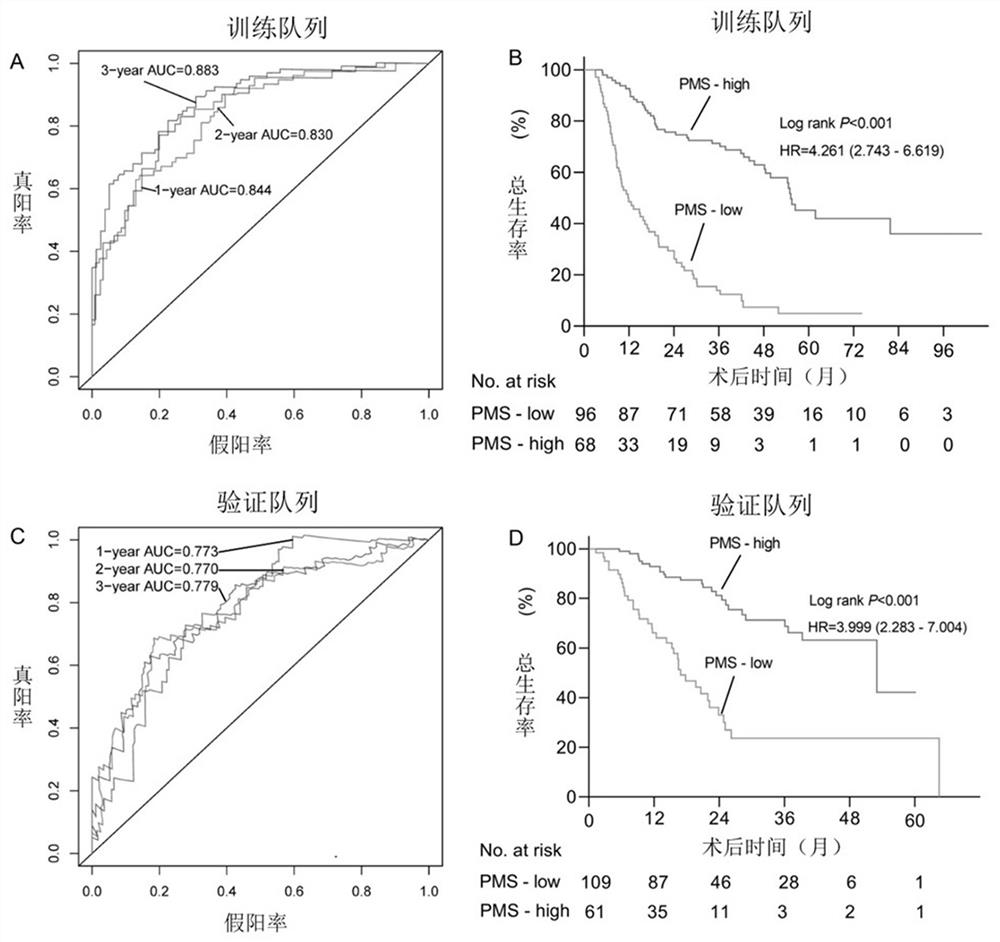
Construction method of prognosis evaluation model of intrahepatic cholangiocarcinoma patient
ActiveCN112270992AStrong correlationImprove accuracyHealth-index calculationMicrobiological testing/measurementDNA methylationIntrahepatic Cholangiocarcinoma
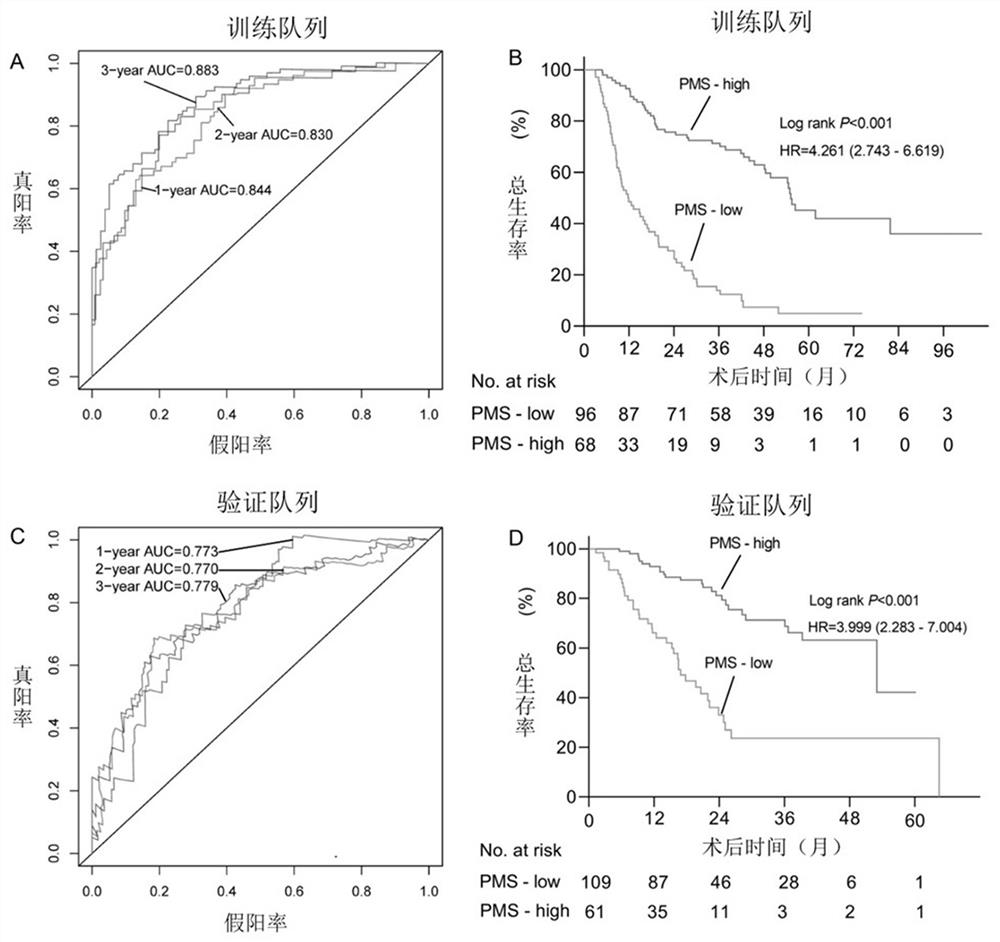
The invention provides a construction method of a model for predicting the survival rate of a patient with intrahepatic cholangiocarcinoma. The construction method comprises the following steps of 1)providing DNA methylation sequencing data of the intrahepatic cholangiocarcinoma; 2) analyzing the DNA methylation sequencing data, and screening out a plurality of related promoter regions of the intrahepatic cholangiocarcinoma; and 3) establishing a model for predicting the survival rate of the intrahepatic cholangiocarcinoma patient by utilizing the plurality of related promoter regions of theintrahepatic cholangiocarcinoma. By conducting DNA methylation prognostic labeling on intrahepatic cholangiocarcinoma patients, the DNA methylation markers serve as independent variables, the patientsare distinguished into a high death rate group and a low death risk group, and experimental verification shows that the construction method of the model can be used for predicting the total postoperative survival rate of the intrahepatic cholangiocarcinoma patients, and can be used for predicting the postoperative survival rate of the intrahepatic cholangiocarcinoma patients; an individualized adjuvant therapy strategy is provided for a patient with the intrahepatic cholangiocarcinoma.
Owner:浙江高美基因科技有限公司
Application of protein muc13 in preparation of reagents for diagnosing intrahepatic cholangiocarcinoma
ActiveCN108982854BStrong specificityIncreased sensitivityMaterial analysisSerum markersIntrahepatic Cholangiocarcinoma
The invention discloses the application of protein MUC13 in the preparation of reagents for diagnosing intrahepatic cholangiocarcinoma. The present invention utilizes the advantages of high-throughput and rapid analysis of the human proteome chip to analyze the relevant serum of patients with intrahepatic cholangiocarcinoma, compare the differences between the samples of intrahepatic cholangiocarcinoma patients and healthy people in a short period of time, and find that the MUC13 protein The expression level of MUC13 in patients with intrahepatic cholangiocarcinoma was significantly higher than that in healthy people, suggesting that MUC13 protein can be used as a candidate serum biomarker for intrahepatic cholangiocarcinoma for early diagnosis and effective treatment of intrahepatic cholangiocarcinoma. Tests have proved that the serum marker MUC13 provided by the present invention has a specificity of 90%, a sensitivity of 91.67%, and has the characteristics of high specificity and high sensitivity. Further, the present invention also provides a sensitive, safe, reliable, and easy-to-operate commercial kit, which can be used to qualitatively measure the level of anti-MUC13 IgG antibody in human serum, which is helpful for the early diagnosis of intrahepatic cholangiocarcinoma.
Owner:HARBIN MEDICAL UNIVERSITY
FISH probe set for detecting FGFR2 fusion and application thereof
ActiveCN109988837AConducive to individualized targeted therapyReduced individualized targeted therapyMicrobiological testing/measurementDNA/RNA fragmentationPatient survivalBac clone
The invention discloses an FISH probe set for detecting FGFR2 fusion and application thereof. The probe set comprises a 5' end probe set and a 3' end probe set; the 5' end probe set adopts BAC of a nucleotide of one side of an FGFR2 gene telomere as a template through cloning and is obtained by amplifying random primers, and each probe is modified with a first reporter group; the 3' end probe setis obtained by cloning the BAC of the nucleotide sequence of the centromere side of the FGFR2 gene into a template and amplifying the random primers, and each probe is modified with a second reportergroup. The set has the advantages of rapid and simple operation, accurate and reliable detection result and accurate detection of all fusion types of the FGFR2 gene, provides a reference for the treatment of intrahepatic cholangiocarcinoma patients, is beneficial to individualized targeted therapy of the patients, reduces drug side effects, improves patient survival quality, reduces the burden onfamilies and society, and is suitable for large-scale promotion and application.
Owner:AMOYDX BIOTECHNOLOGY RES CENT CO LTD
Intrahepatic cholangiocarcinoma patient prognosis lifetime prediction system
ActiveCN112289450AForecast stabilityImprove accuracyMedical data miningHealth-index calculationIntrahepatic CholangiocarcinomaIntrahepatic bile duct cancer
The invention provides an intrahepatic cholangiocarcinoma patient prognosis lifetime prediction system, which comprises an acquisition module and a prediction module, and is characterized in that theacquisition module and the prediction module are connected in a wireless and / or wired mode. The prediction system can predict the prognostic lifetime of the intrahepatic cholangiocarcinoma patient byadopting the promoter methylation score as a unique variable, can predict the prognostic lifetime of the intrahepatic cholangiocarcinoma patient based on multiple variables including the promoter methylation score, and has the advantages of high accuracy, strong reliability and stable prediction.
Owner:浙江高美生物科技有限公司
A kind of human intrahepatic cholangiocarcinoma cell line and its application
InactiveCN104560878BAlgebraically clearNo cross contaminationMicrobiological testing/measurementMicroorganism based processesIntrahepatic CholangiocarcinomaIn vivo
The invention relates to a human intrahepatic duct cancer cell line and an application thereof. The human intrahepatic duct cancer cell line LICCF is established according to the invention, is clear and definite in algebra, does not have cross contamination, shows unique sensitivity to an inhibitor of a PI3K / mTOR passage and an inhibitor of an MEK1 / 2 passage, has high drug resistance to 5-FU, cis-platinum and FGFR inhibitors, and can be used for establishing in vivo and in vitro tumor drug experiment and drug resistance models, so that an experimental basis is provided for revealing a human intrahepatic duct cancer drug resistance mechanism, reversing the appearance of drug resistance, developing new anticancer drugs and screening human intrahepatic duct cancer related biological markers.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Marker for evaluating gemcitabine chemosensitivity of intrahepatic cholangiocarcinoma and application of marker
The invention discloses a marker for evaluating the gemcitabine chemosensitivity and / or prognosis of intrahepatic cholangiocarcinoma and application of the marker. The marker for evaluating the gemcitabine chemosensitivity and / or prognosis of intrahepatic cholangiocarcinoma is CXCR3 expressed on the surfaces of peripheral blood leukocytes or immune cells in cholangiocarcinoma tissues of an intrahepatic cholangiocarcinoma patient. A kit for evaluating the gemcitabine chemosensitivity and / or prognosis of intrahepatic cholangiocarcinoma comprises a reagent related to detection of the expression quantity of the CXCR3 protein. A method for evaluating the gemcitabine chemosensitivity and / or prognosis of intrahepatic cholangiocarcinoma comprises the following steps: (1) detecting the expression quantity of CXCR3 on the surfaces of peripheral blood leukocytes or immune cells in cholangiocarcinoma tissues of an intrahepatic cholangiocarcinoma patient; and (2) comparing the expression quantity with a preset CXCR3 protein expression quantity threshold value, evaluating that the patient is sensitive to gemcitabine chemotherapy and / or good in prognosis of gemcitabine chemotherapy if the CXCR3 protein expression quantity is higher than the threshold value, and otherwise, evaluating that the patient is insensitive to gemcitabine chemotherapy and / or poor in prognosis of gemcitabine chemotherapy.
Owner:中国人民解放军海军军医大学第三附属医院
A predictive system for prognostic survival of patients with intrahepatic cholangiocarcinoma
ActiveCN112289450BForecast stabilityImprove accuracyMedical data miningHealth-index calculationIntrahepatic CholangiocarcinomaOncology
The present application provides a prediction system for prognosis and survival of patients with intrahepatic cholangiocarcinoma, including: an acquisition module and a prediction module, and the acquisition module and the prediction module are connected in a wireless and / or wired manner. The prediction system can use the promoter methylation score as the only variable to predict the survival of patients with intrahepatic cholangiocarcinoma, and can also predict the survival of intrahepatic cholangiocarcinoma based on multiple variables including the promoter methylation score. The prognostic survival period of cancer patients has the advantages of high accuracy, strong reliability, and stable prediction.
Owner:浙江高美生物科技有限公司
Prognostic biomarkers and detection kits for patients with intrahepatic cholangiocarcinoma
ActiveCN112280868BRealize non-invasive testingWith non-invasive detectionMicrobiological testing/measurementDNA/RNA fragmentationIntrahepatic CholangiocarcinomaBiologic marker
The present application discloses a prognostic biomarker and a detection kit for patients with intrahepatic cholangiocarcinoma. Using the set of polynucleotide sequences shown in SEQ ID NO.1‑SEQ ID NO.24 as biomarkers, methylation analysis was performed on these promoter regions, and an effective methylation scoring model was constructed to realize liver Accurate assessment of prognostic survival in patients with internal cholangiocarcinoma. This biomarker can be used as a single predictor of prognosis in patients with intrahepatic cholangiocarcinoma or as a predictor of survival in patients with intrahepatic cholangiocarcinoma based on multiple variables including gene promoter methylation scores.
Owner:浙江高美生物科技有限公司
Construction method of prognosis assessment model for patients with intrahepatic cholangiocarcinoma
ActiveCN112270992BStrong correlationImprove accuracyHealth-index calculationMicrobiological testing/measurementDNA methylationIntrahepatic Cholangiocarcinoma
Owner:浙江高美基因科技有限公司
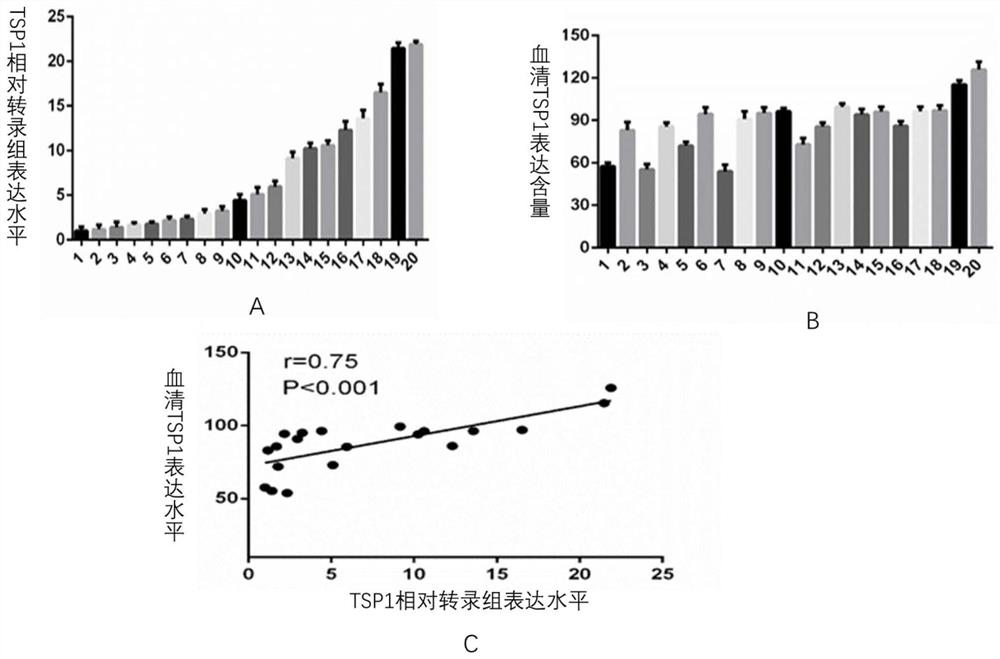
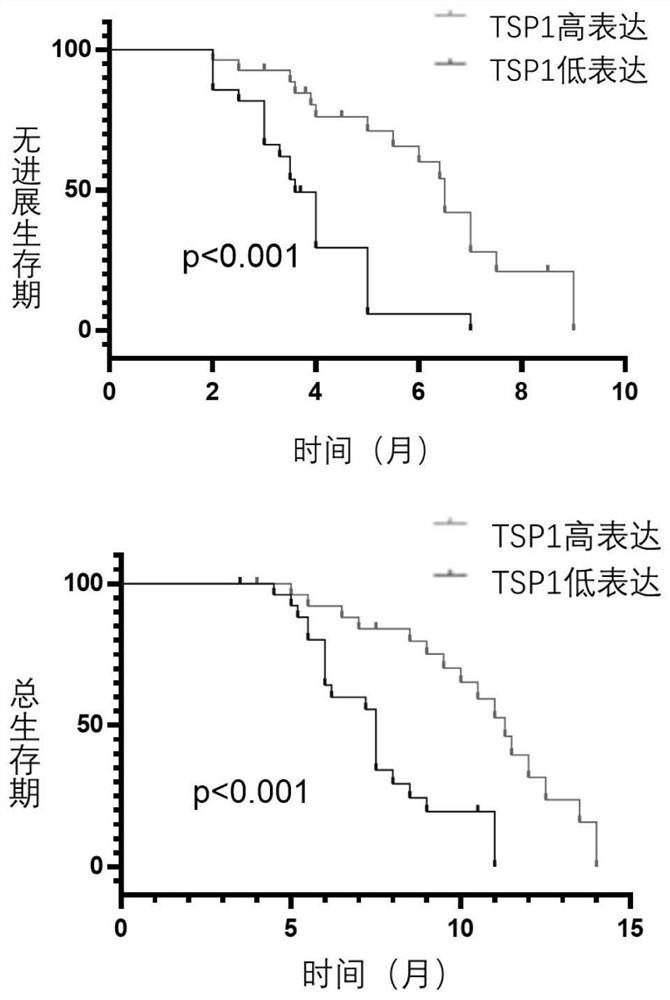
Application of human thrombin-sensitive protein-1 in preparation of kit for predicting chemotherapy effect of intrahepatic cholangiocarcinoma
The invention relates to the technical field of medical biological detection, and concretely relates to application of human thrombin-sensitive protein-1 in preparation of a kit for predicting the chemotherapy effect of intrahepatic cholangiocarcinoma. The application has the advantages that the protein chip is used for screening out the protein with expression difference in tissues of patients of an intrahepatic cholangiocarcinoma (ICC) chemotherapy drug-resistant group and an ICC chemotherapy sensitive group in the middle and advanced stages, the protein can be detected in serum, and it is found that the thrombin-sensitive protein-1 has the most obvious difference in two groups of patients and has a very high expression level in sensitive group patients. The invention provides the application of human thrombin-sensitive protein-1 as a serum marker for determining whether chemotherapy of an intrahepatic cholangiocarcinoma patient is sensitive or not. The expression level of the human thrombin-sensitive protein-1 is closely related to survival prognosis of the patient. The application provides a new clinical means for serological diagnosis.
Owner:上海柏运医疗器材有限公司
A fish probe set for detecting fgfr2 fusion and its application
ActiveCN109988837BConducive to individualized targeted therapyReduced individualized targeted therapyMicrobiological testing/measurementDNA/RNA fragmentationIntrahepatic CholangiocarcinomaSide effect
The invention discloses a FISH probe set for detecting FGFR2 fusion and its application, including a 5' end probe set and a 3' end probe set; The BAC clone of the sequence is used as a template and amplified with random primers, in which each probe is modified with the first reporter group; the 3' end probe group is based on the BAC of the nucleotide sequence on the centromere side of the FGFR2 gene Clones are used as templates and amplified with random primers in which each probe is modified with a second reporter group. The invention is quick and easy to operate, accurate and reliable in detection results, can accurately detect all fusion types of the FGFR2 gene, provides a reference for the treatment plan of patients with intrahepatic cholangiocarcinoma, is beneficial to individualized targeted therapy for patients, reduces drug side effects, and improves patient survival Quality, reduce family and social burden, suitable for large-scale promotion and application.
Owner:AMOYDX BIOTECHNOLOGY RES CENT CO LTD
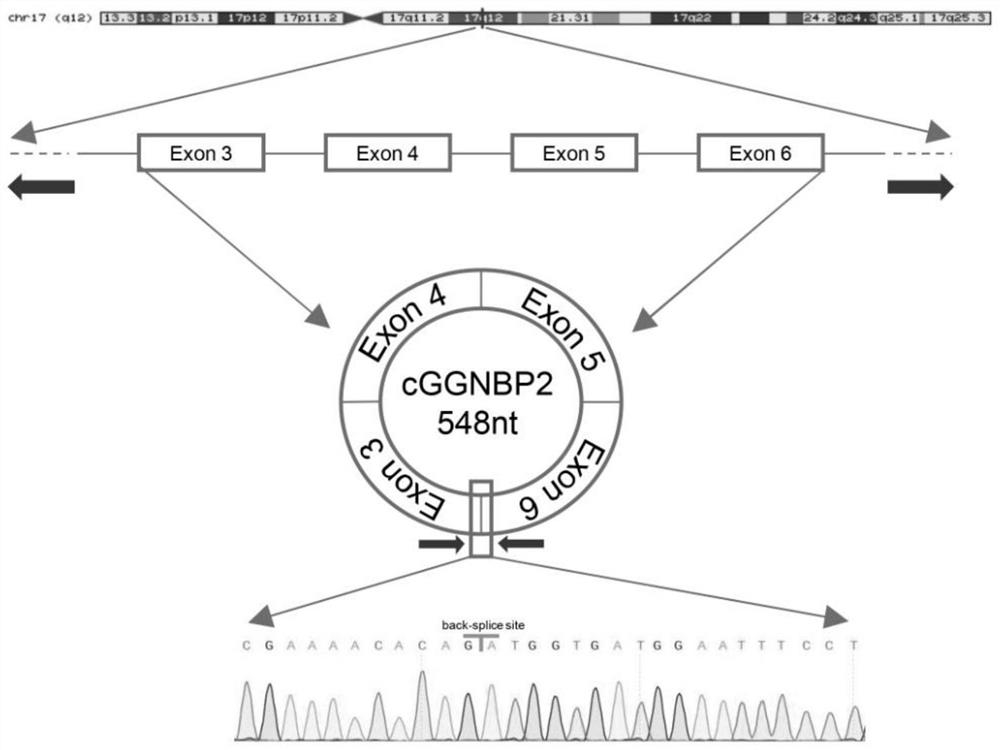
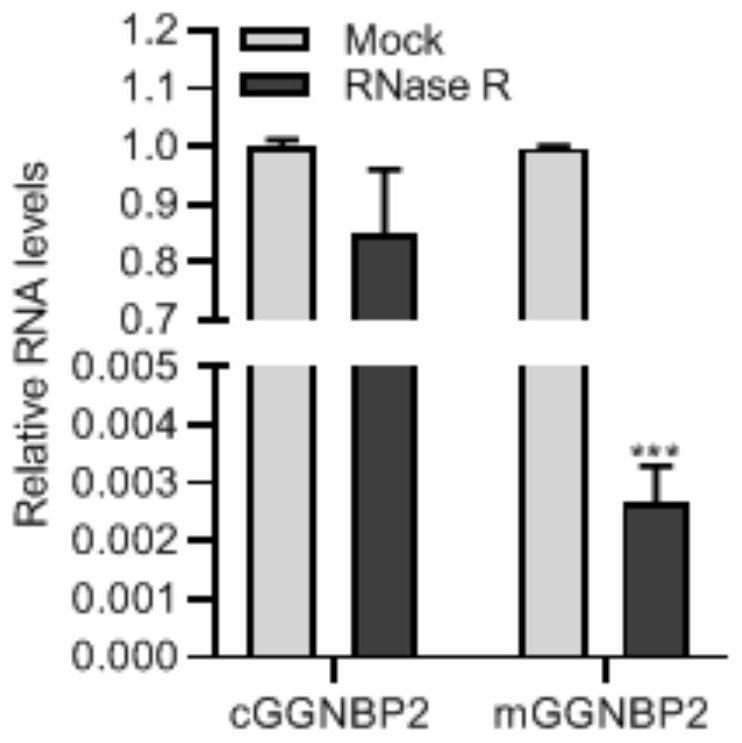
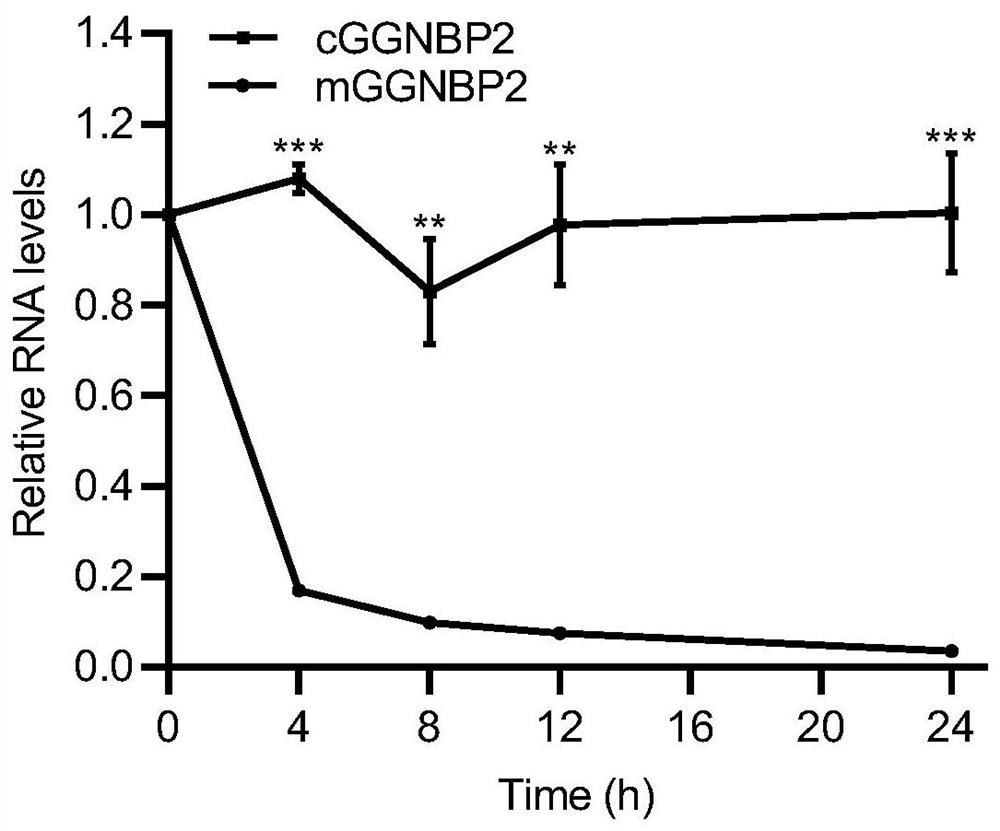
Application of circular RNA cGGNBP2
ActiveCN114032306AReduce expressionInhibition of proliferative abilityMicrobiological testing/measurementAgainst vector-borne diseasesIntrahepatic CholangiocarcinomaPharmaceutical drug
The invention discloses application of circular RNA cGGNBP2, and provides a kit for diagnosing intrahepatic cholangiocarcinoma and a pharmaceutical composition for treating the intrahepatic cholangiocarcinoma based on the function and mechanism research of the inventor on the circular RNA cGGNBP2 (circBase ID: hsa_circ_0003930) in growth and metastasis of hepatic cholangiocarcinoma cells. The invention also discloses application of the circular RNA cGGNBP2 as a therapeutic target in prognosis, diagnosis and treatment of intrahepatic cholangiocellular carcinoma.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Products and methods for the identification of hepatocellular carcinoma and intrahepatic cholangiocarcinoma based on MANF as a marker
ActiveCN106645725BImproved prognosisImprove the quality of lifeMaterial analysisPediatric Hepatocellular CarcinomaIntrahepatic Cholangiocarcinoma
The invention discloses a product and a method for identifying hepatocellular carcinoma and intrahepatic cholangiocarcinoma based on an MANF as a marker. The invention provides an application of a system for detecting the expression quantity of the MANF in preparation of products for identification or supplementary identification of hepatocellular carcinoma patients and intrahepatic cholangiocarcinoma patients. The hepatocellular carcinoma and the intrahepatic cholangiocarcinoma are identified by taking the MANF as the marker and detecting the expression level of the MANF in a primary hepatocellular carcinoma tissue, a method and an index for effectively identifying the hepatocellular carcinoma and the intrahepatic cholangiocarcinoma are built, the sensitivity is 92.5% and the specificity is 74.7%. A foundation is laid for targeted appropriate treatment and improvement of prognosis of the patients to improve the living quality of the patients and to reduce the economic burden of the patients.
Owner:ANHUI MEDICAL UNIV
Application of SLC12A5 and inhibitor thereof
ActiveCN113730582APrevent proliferationInhibition of clonogenicityOrganic active ingredientsAntineoplastic agentsIntrahepatic CholangiocarcinomaMolecular biology
The invention relates to the technical field of biomedical engineering, and discloses application of SLC12A5 and an inhibitor of the SLC12A5. The biological function of the SLC12A5 is inhibited through the specific inhibitor, and compared with a control group, the SLC12A5 inhibitor can significantly inhibit bile duct cancer cell proliferation and clone formation rate, and also can significantly inhibit subcutaneous tumor growth, so that therefore, a new way for preparing the medicine for treating or improving the intrahepatic cholangiocarcinoma and treating the intrahepatic cholangiocarcinoma can be provided by taking the SLC12A5 as the target spot.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Diagnosis method and diagnosis kit for intrahepatic cholangiocarcinoma
ActiveCN111073980AFast waySimple methodMicrobiological testing/measurementIntrahepatic CholangiocarcinomaPharmaceutical drug
The invention provides a diagnosis method and diagnosis kit for intrahepatic cholangiocarcinoma. The kit comprises primers and probe aiming at mutant type glypican 3 (GPC3) complementary DNA (cDNA). According to the diagnosis method and the diagnosis kit, experiments show that the glypican 3 can mutate in the intrahepatic cholangiocarcinoma; the mutation has specificity in the intrahepatic cholangiocarcinoma, and therefore, the mutant GPC3 cDNA can be used as a specific marker for diagnosing and treating an acute liver injury with drugs. Based on the above, the invention provides a new hepatocyte diagnosis method and kit.
Owner:MUDANJIANG MEDICAL UNIV
Method for detecting and distinguishing intrahepatic cholangiocarcinoma
ActiveUS8557602B2Difficult to differentiateHigh-precision detectionLibrary screeningMaterial analysis by optical meansAbnormal tissue growthIntrahepatic Cholangiocarcinoma
Disclosed are a method for early, sensitively and reliably detecting and distinguishing intrahepatic cholangiocarcinoma in a malignant tumor occurring primarily in the liver in a simple way, and a kit thereof. In the method, a glycan biomarker consisting of a lectin WFA (Wisteria floribunda Agglutinin)-binding glycoprotein derived from intrahepatic cholangiocarcinoma is used as a cancer marker to detect intrahepatic cholangiocarcinoma by detecting the cancer marker in a test specimen. The method for detecting intrahepatic cholangiocarcinoma can clearly differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma and enables early detection and determination with a performance clinically acceptable in terms of applicability, sensitivity and precision.
Owner:NAT INST OF ADVANCED IND SCI & TECH +1
Application of MMP7, CTSE or LAMC2 proteins in preparation of intrahepatic cholangiocarcinoma diagnostic reagent
ActiveCN112129954ABiological material analysisBiological testingDiseaseIntrahepatic Cholangiocarcinoma
The invention relates to the technical field of medical bioengineering, and aims to provide an application of MMP7, CTSE or LAMC2 proteins in preparation of an intrahepatic cholangiocarcinoma diagnosis reagent. The application is used for preparing a reagent capable of identifying and diagnosing intrahepatic cholangiocarcinoma and distinguishing primary hepatocellular carcinoma by using at least one of the MMP7, the CTSE or the LAMC2 proteins, which means the identification and the distinguishing of the hepatocellular carcinoma and the intrahepatic cholangiocarcinoma are achieved by using thethree proteins independently or in a combined manner. According to the application of the MMP7, the CTSE or the LAMC2 proteins in preparation of the intrahepatic cholangiocarcinoma diagnosis reagent,the reagent or a kit product is prepared from the three proteins, the expression level of the proteins in blood is detected by an immunological related method and is used as a basis for diagnosing anintrahepatic cholangiocarcinoma marker. The application of the MMP7, the CTSE or the LAMC2 proteins in preparation of the intrahepatic cholangiocarcinoma diagnosis reagent can accurately and specifically judge the possibility of the intrahepatic cholangiocarcinoma, benign biliary diseases, the hepatocellular carcinoma and health status of a subject, and provides a basis of the next diagnosis and treatment scheme for doctors.
Owner:杭州道合天孚生物科技有限公司
A diagnostic method and diagnostic kit for intrahepatic cholangiocarcinoma
ActiveCN111073980BFast waySimple methodMicrobiological testing/measurementIntrahepatic CholangiocarcinomaHepatocyte
The invention provides a diagnosis method and diagnosis kit for intrahepatic cholangiocarcinoma. The kit comprises primers and probe aiming at mutant type glypican 3 (GPC3) complementary DNA (cDNA). According to the diagnosis method and the diagnosis kit, experiments show that the glypican 3 can mutate in the intrahepatic cholangiocarcinoma; the mutation has specificity in the intrahepatic cholangiocarcinoma, and therefore, the mutant GPC3 cDNA can be used as a specific marker for diagnosing and treating an acute liver injury with drugs. Based on the above, the invention provides a new hepatocyte diagnosis method and kit.
Owner:MUDANJIANG MEDICAL UNIV
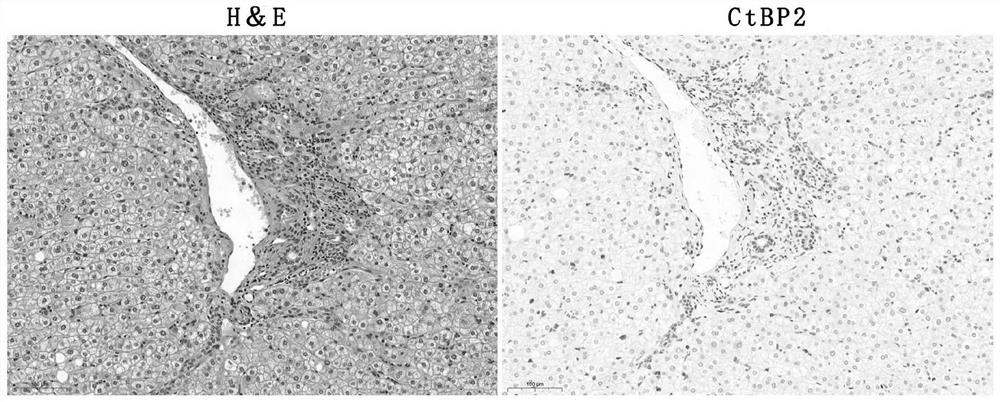
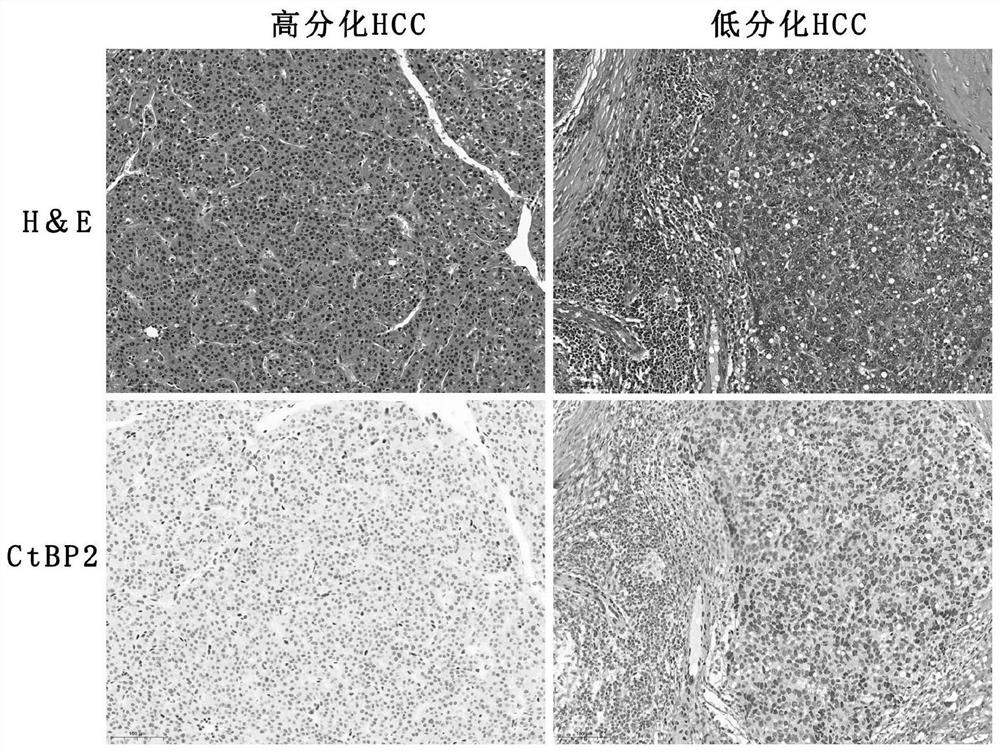
Application of ctbp2 in the preparation of diagnostic products for hepatocellular carcinoma and intrahepatic cholangiocarcinoma
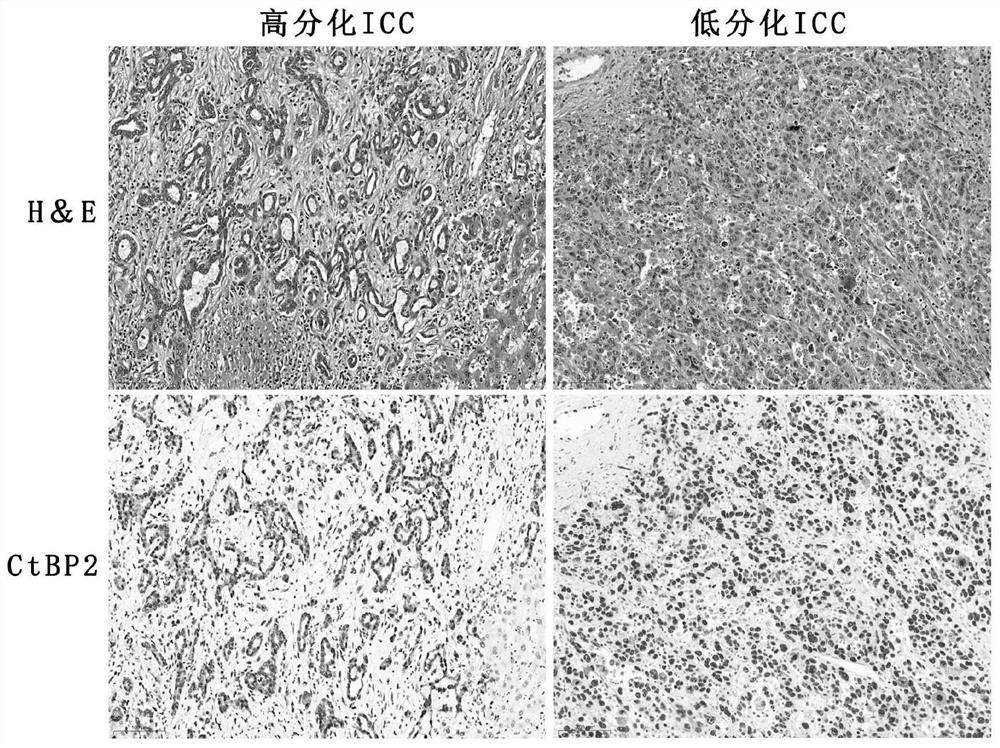
The invention discloses an application of using CtBP2 as a marker in the preparation of diagnostic products for hepatocellular carcinoma and intrahepatic cholangiocarcinoma, and belongs to the technical field of biomedicine. The research results of the present invention show that most high-moderately differentiated HCCs are negative for CtBP2, and only a few poorly differentiated HCCs are positive; while in ICC, CtBP2 is diffusely strongly positive. Therefore, CtBP2 can be used as a marker to prepare related products for the identification and diagnosis of hepatocellular carcinoma and intrahepatic cholangiocarcinoma, thereby providing a new method for the identification, diagnosis or drug efficacy evaluation of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. objective indicators.
Owner:NANTONG TUMOR HOSPITAL
Application of substances targeting manf in the preparation of products for the treatment of intrahepatic cholangiocarcinoma
ActiveCN109364249BPrevent proliferationInhibit migrationOrganic active ingredientsDigestive systemIntrahepatic CholangiocarcinomaBiochemistry
The invention discloses the application of a substance targeting MANF in the preparation of a product for treating intrahepatic cholangiocarcinoma. Experiments have proved that the substances that inhibit the expression of MANF in cells or the substances that inhibit the activity of MANF in cells can effectively inhibit the proliferation, migration and / or invasion of cholangiocarcinoma cells, inhibit the growth of cholangiocarcinoma tumors, and can be used to treat intrahepatic cholangiocarcinoma or / and prevent Intrahepatic cholangiocarcinoma.
Owner:ANHUI MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com