Application of MMP7, CTSE or LAMC2 proteins in preparation of intrahepatic cholangiocarcinoma diagnostic reagent
A technology for inner bile duct and cell carcinoma, which is applied in the field of medical bioengineering and can solve the problems of unsatisfactory sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
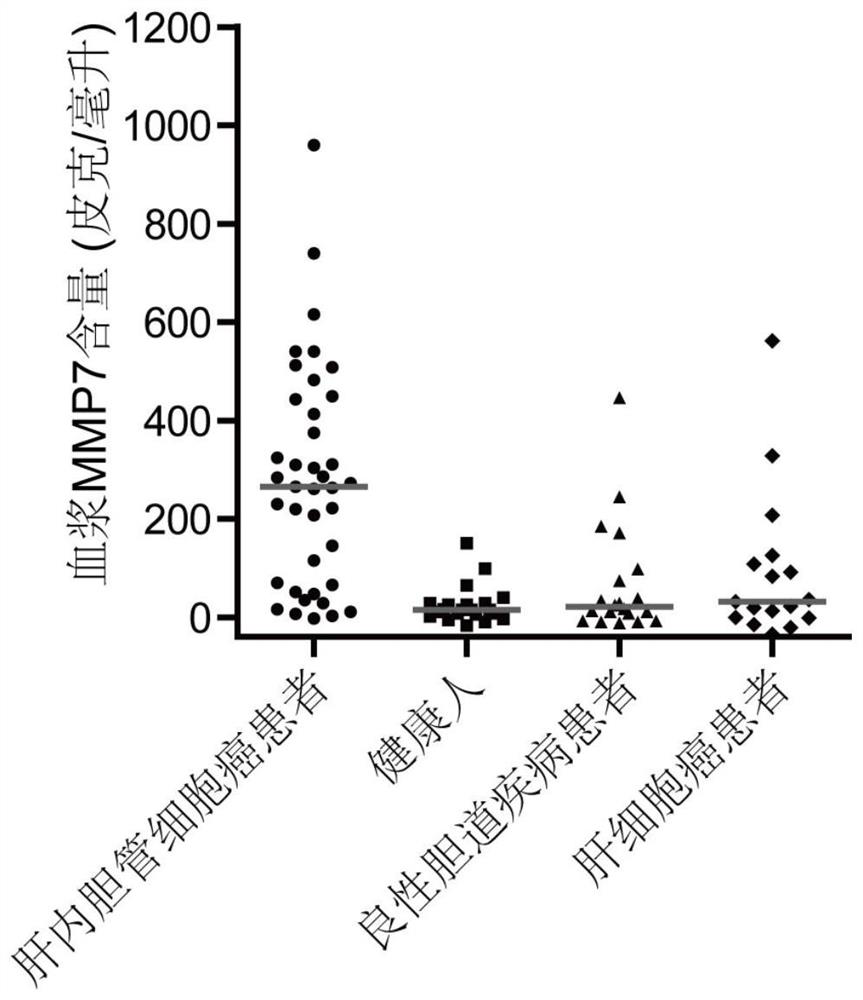
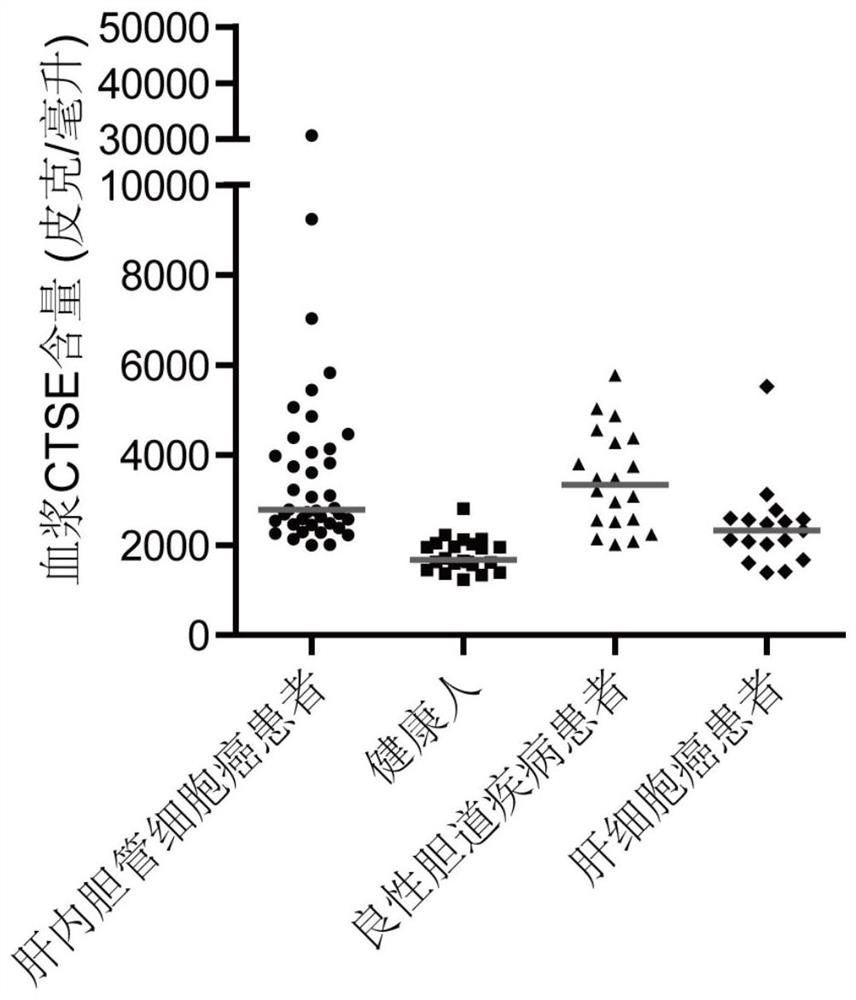
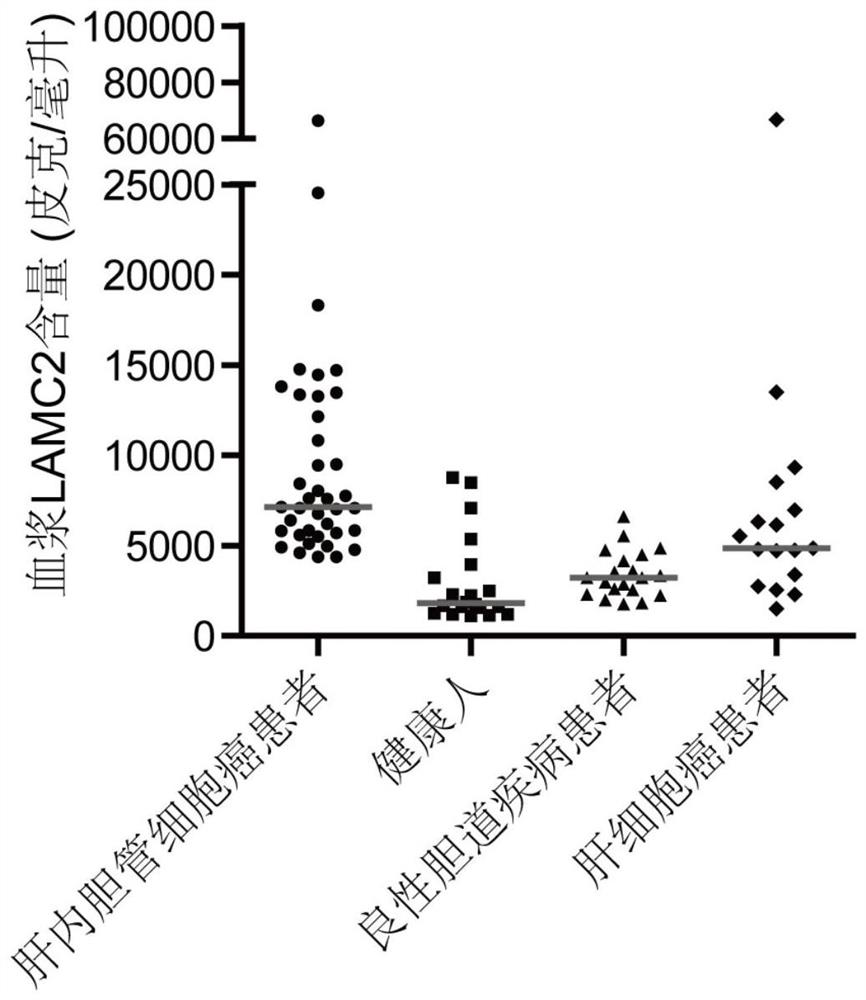
[0049] Example 1: The detection of plasma MMP7, CTSE, and LAMC2 helps to improve the clinical diagnostic ability of intrahepatic cholangiocarcinoma.
[0050] A total of 98 volunteers were selected in a hospital, including 39 patients with intrahepatic cholangiocarcinoma, 22 healthy people, 20 patients with benign biliary tract disease and 17 patients with hepatocellular carcinoma. Extract biological samples from volunteers, record characteristic parameters, and use kits for specific detection. The characteristic parameters of the test group are shown in Table 1.
[0051] Table 1. Clinical characteristics of 39 patients with intrahepatic cholangiocarcinoma, 17 patients with hepatocellular carcinoma, and 20 patients with benign biliary disease
[0052] a: Fisher's exact test; b: chi-square test; c: variance test
[0053]
[0054]
[0055]
[0056] In this experiment, the commercially available antibody sandwich method was used to detect the three proteins. Standard pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com