Diagnosis method and diagnosis kit for intrahepatic cholangiocarcinoma
The technology of a kit and a detection kit, which is applied in the field of molecular biology, can solve the problems of the undisclosed expression of GPC3 in intrahepatic cholangiocarcinoma, and achieve the effects of high sensitivity, simple method, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Detection of mutant truncated GPC3 proteoglycans
[0031] With the consent of the patient, the inventor of the present application took a cancer cell tissue sample from a patient diagnosed with intrahepatic cholangiocarcinoma for protein analysis, extracted the total protein by cell disruption technology, and obtained the GPC3 proteoglycan in it by antibody purification technology. Protein structure analysis found that the GPC3 proteoglycan structure was different from the known GPC3 proteoglycan structure in hepatocellular carcinoma. The GPC3 protein was sent out for protein sequencing, and its sequence is shown in SEQ ID No.5. Sequence comparison found that compared with the known high expression of GPC3 protein in hepatocellular carcinoma (as shown in SEQ ID No.4, NCBI database number: XP_018874999.1), the GPC3 proteoglycan in the above intrahepatic cholangiocarcinoma samples There are two mutation sites, Y314S and M316I, and 33 amino acid sequences at th...
Embodiment 2
[0033] Embodiment 2 Specific primers and probe design and PCR amplification method
[0034] In response to the above findings, the inventors confirmed the cDNA sequence of the above-mentioned mutant truncated GPC3 protein through a genetic screening analysis method, and designed primers and probes for the above-mentioned mutant truncated GPC3 proteoglycan mutation site for the sequence, The specific sequences are respectively shown in SEQ ID No.1-3. The two ends of the probe shown in SEQ ID No.3 are respectively labeled with a fluorescent reporter group FAM and a fluorescent quencher group TAMRA. The above primers can amplify the cDNA sequence containing the mutation site, and the probe
[0035] The method for target sequence-specific amplification detection using the above primers and probes is as follows:
[0036] (1) extract the total RNA in the sample tissue (peripheral blood), and reverse transcribe it into cDNA;
[0037] (2) using the cDNA in step (1) as a template, usi...
Embodiment 3
[0043] Embodiment 3 clinical examination test
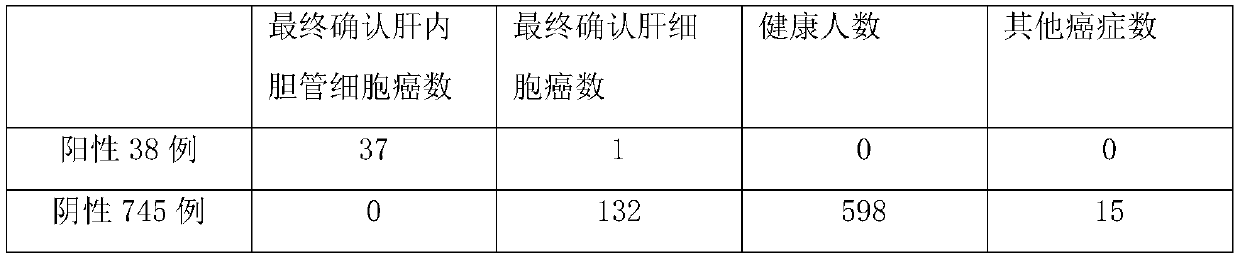
[0044] With the consent of the patient, the peripheral blood of 783 patients undergoing screening and detection of hepatocellular carcinoma or intrahepatic cholangiocarcinoma was tested using the method of the above-mentioned embodiment 2. Among them, the method described in this application showed positive results in 38 cases and negative results in 745 cases. Example; the above positive and negative results were verified separately, and the final confirmation test results are shown in the following table:
[0045]
[0046] It can be seen that the mutant truncated GPC3 proteoglycan described in the present application has good specificity for intrahepatic cholangiocarcinoma, and only 1 case was detected in 38 positive patients using the primers and probes of the present invention. Hepatocellular carcinoma was finally confirmed, and among the 745 negative patients, 132 were diagnosed as hepatocellular carcinoma, and 15 were ot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com