Preparation method and application of tumour cell specific polyclonal T cells
A tumor cell, specific technology, applied in the fields of molecular biology and cellular immunology, can solve the problems of high cost, time-consuming and laborious, limited application scope, etc., and achieve the effect of reducing production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
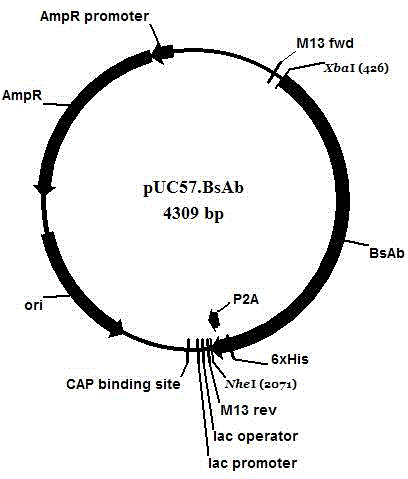
[0050] Example 1: This example provides a method for constructing a recombinant lentiviral expression vector capable of stably expressing CD20 on the cell membrane surface and secreting anti-CD20×CD3, including the following steps:
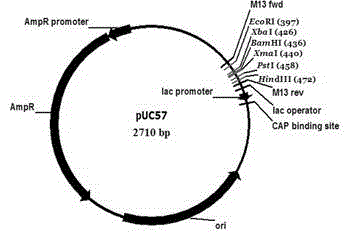
[0051] (1) This example provides a method for constructing a pUC57 recombinant plasmid vector containing CD20×CD3 gene
[0052] (1a) Synthesize the sequentially connected Xba I restriction site-the base sequence encoding the signal peptide of the immunoglobulin κ chain (SEQ ID NO:1)-the base sequence encoding the Flag tag (SEQ ID NO:1) by means of whole gene sequence synthesis NO:2)-BsAb base sequence (SEQ ID NO:3)-base sequence encoding histidine His6 tag (SEQ ID NO:4)-P2A base sequence (SEQ ID NO:5)-NheI enzyme The cleavage site, the sequentially connected base sequences described in this example are only one of the double strands of the gene (that is, the DNA fragment) synthesized by the whole gene, and the other strand is complementary to the ...
Embodiment 2
[0071] Example 2: This example provides a method for constructing HepG2 cells (named: HepG2-BsAb.TAg) that can stably express CD20 on the cell membrane surface and secrete CD20×CD3, including the following steps:
[0072] (1) Recombinant lentiviral particles transfect HepG2
[0073] HepG2 cells were cultured according to conventional methods, and the cell density was adjusted to 5×10 with DMEM / F12 complete medium containing 100 mL / L fetal bovine serum. 5 / mL, trypsinize HepG2 1 day before transfection, and re-inoculate in the culture flask; when the cell growth confluence reaches 70%-80%, replace with a new culture, and add 20 μL of recombinant lentiviral particles. After 4 hours of infection, the culture medium was changed and the culture was continued, and the empty vector without the target gene was used as the control.
[0074] (2) Screening for stable expression strains
[0075] 48 h after infection, puromycin was added at a final concentration of 2 g / L to screen for st...
Embodiment 3
[0083] Example 3: This example provides a method for preparing HepG2-BsAb.TAg-mediated TSPT cells, including the following steps:
[0084] (1) Peripheral venous blood was collected from patients, and mononuclear cells were obtained by density gradient centrifugation of Ficoll-diatrizoate glucosamine. The specific steps are: double-dilute the precipitated blood cells with normal saline, add human lymphocyte separation solution and diluted blood into the centrifuge tube at a ratio of 1:2, centrifuge at 2000 rpm for 20 min, carefully absorb the buffy coat layer, and wash with normal saline Centrifuge twice at 1600 rpm and 1300 rpm for 7 min to obtain PBMC.
[0085] (2) Resuspend the above PBMC in commercial serum-free medium such as PAA? or GT-T551?, and adjust the cell density to 1×10 6 / ml, mixed with HepG2-BsAb.TAg of equal volume and density, added rhIL-2 after mixing, the final concentration was 300 IU / ml, and cultured in an incubator at 37°C and 5% CO2; after 48 hours, Cou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com