Preparing method for high-toxicity human Vgamma9Vdelta2 T cells induced by PD-1 antibody
A PD-1 and cell technology, applied in the field of cellular immunology, can solve the problems of limited application and promotion, low number of γδT cells, etc., and achieve the effect of enhancing secretion capacity, improving cytotoxicity, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] Example 1 is to stimulate PBMCs with IL-12 and PD-1 antibodies to obtain a large number of Vγ9Vδ2T cells with high purity and high cytotoxic activity, which specifically includes the following steps:
[0029] 1. Collect the peripheral venous blood of the patient with a 50ml syringe under sterile conditions, and obtain mononuclear cells by centrifugation of polysucrose-diatrizoate according to the density gradient. The specific steps are: centrifuge at 500g / min for 7 minutes, absorb the upper plasma layer, inactivate at 56°C for 30 minutes, then inactivate at 900g / min for 10 minutes, and prepare the supernatant plasma for later use; double-dilute the lower layer of blood cells with normal saline, human lymphocyte separation medium Add the diluted blood to the centrifuge tube at a ratio of 1:2, centrifuge at 680g / min for 20 minutes, absorb the buffy coat, wash twice with normal saline, and centrifuge at 500g / min and 410g / min for 7 minutes respectively. Peripheral blood mo...
example 2
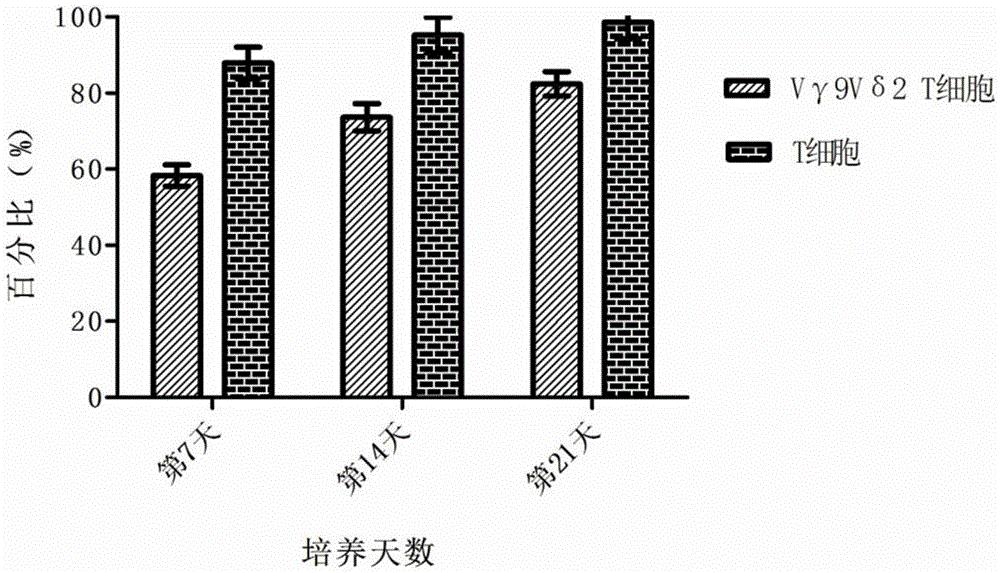
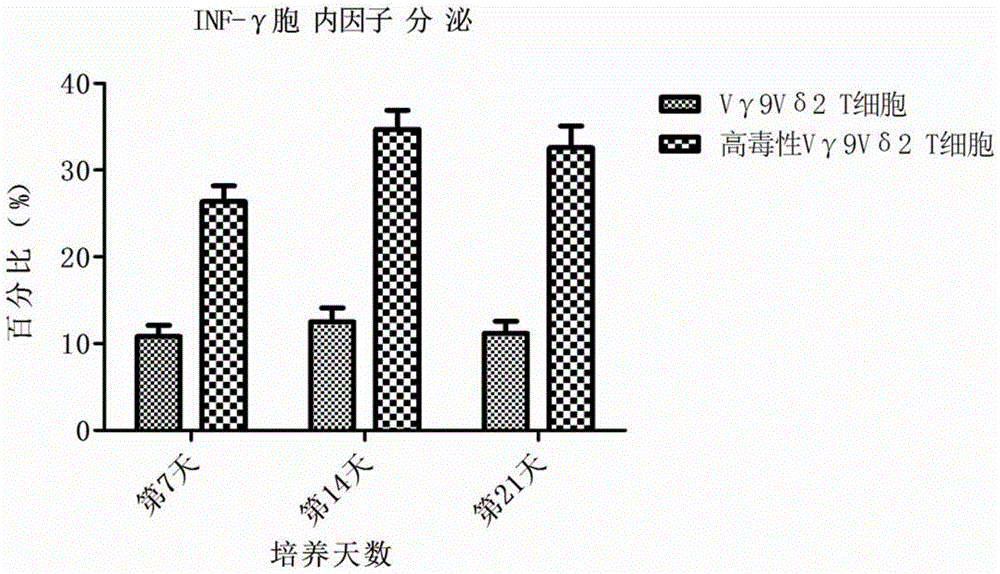
[0032] In Example 2, the morphology, purity, immunophenotype, cytokine secretion and cytotoxic activity of the above-mentioned cultured Vγ9Vδ2T cells were detected. The specific operation includes the following steps:
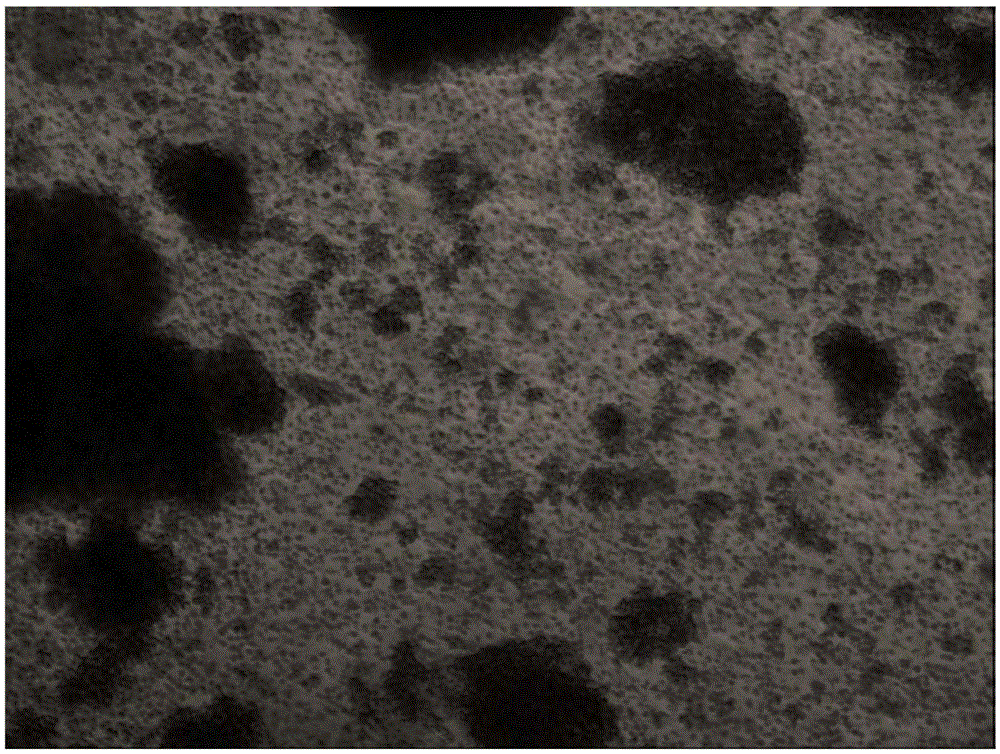
[0033] 1. After 24 hours of cell culture, it can be seen that Vγ9Vδ2T cells sink to the bottom of the culture flask and form small colonies. After 48 hours, the colonies become larger. After 6-11 days of culture, the cell colonies can be seen to increase, increase, and have irregularities. of monocytes. After 11 days of culture, most of the cells increased in volume and were oval or irregular (see appendix figure 1 ), stained with 4% trypan blue, and counted under a microscope, the results showed that the viability of the cells prepared by the present invention was 97.3% (n=10).
[0034] 2. Take the cells on day 0, 7, 14 and 21 respectively, wash the cells twice with flow cytometry buffer, and adjust the cell density to 1×10 6 cell / ml, add 50ul of cell suspe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com