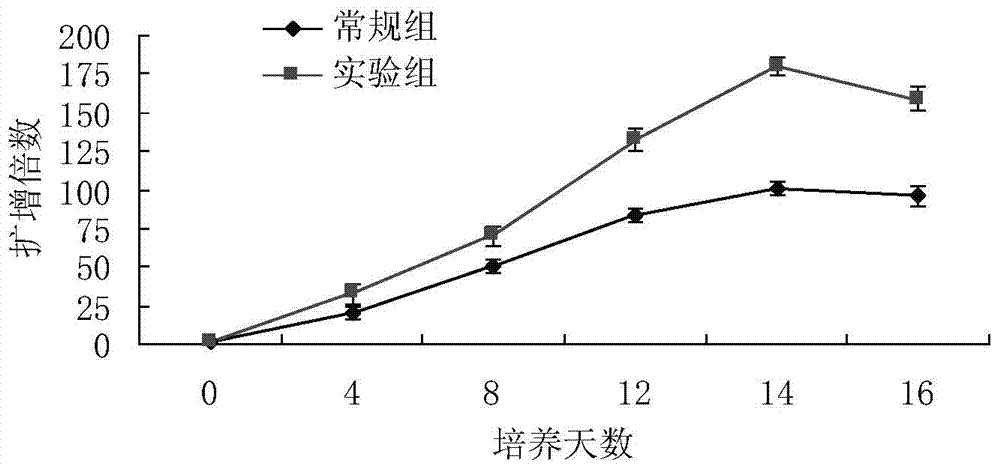
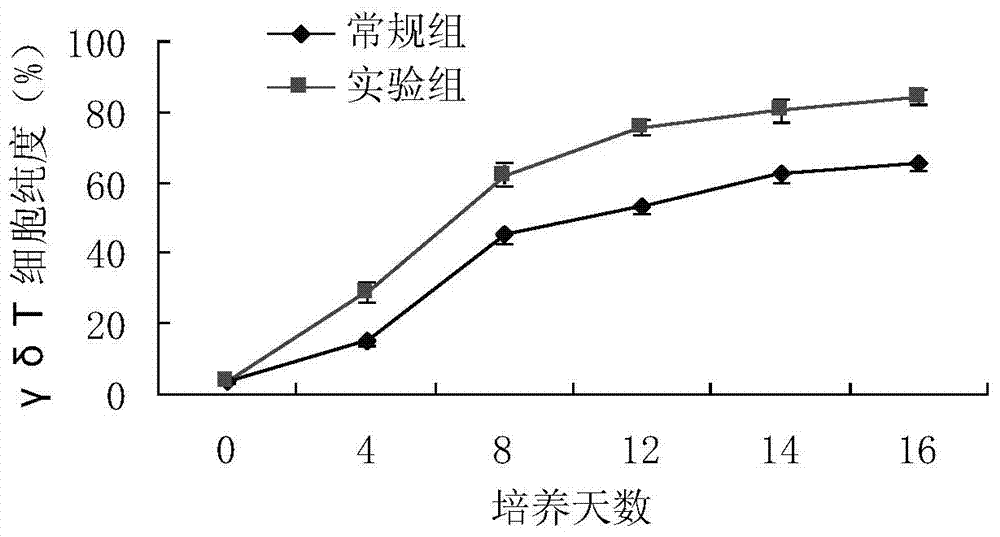
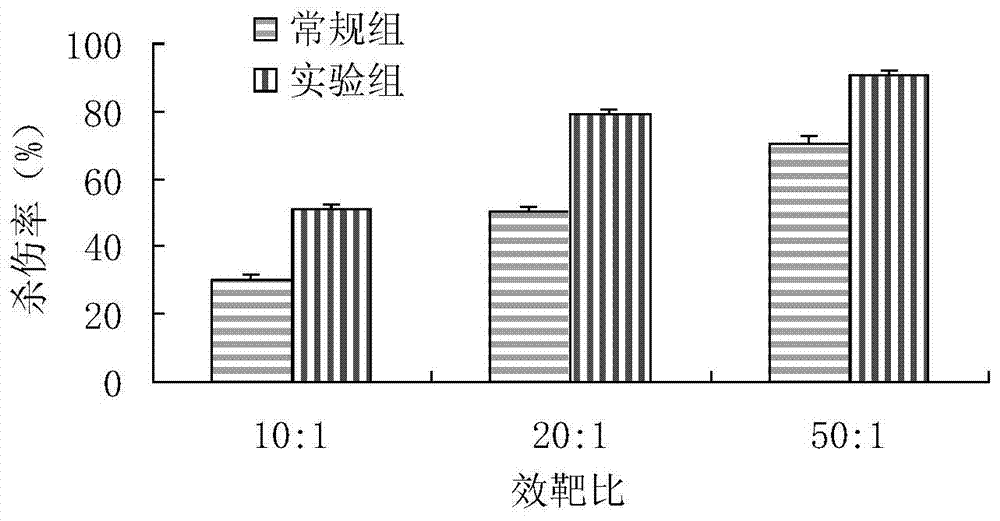
Method used for in vitro amplification of gamma-delta-T cells
An in vitro amplification and cell technology, applied in animal cells, vertebrate cells, blood/immune system cells, etc., can solve problems such as high cost and insufficient amplification multiples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Isolation and culture of peripheral blood mononuclear cells (PBMC)
[0027] The machine collects PBMC, transfers the collected blood sample to a centrifuge tube; centrifuges at 700g for 10 minutes, absorbs the upper layer of plasma for later use; restores the blood sample to its original volume and mixes; slowly adds diluted blood to Ficoll, 900g, and centrifuges for 20 minutes; absorbs Milky white mononuclear cell layer at the separation liquid interface; centrifuged and washed twice and counted; cells were resuspended to 1×10 with γδT initial medium 6 / ml; According to the growth of the cells, replace the medium every 2 to 3 days, and the medium used is a serum-free medium containing 5% autologous plasma. At 37°C, 5% CO 2 According to the growth of the cells, the medium was replaced every 2-3 days, and the cell concentration was controlled at 2.5×10 6 At the same time, various factors were supplemented in full amount: the test group was supplemented with zoledron...
Embodiment 5
[0044] Example 5: Determination of immunological response results of all γδT subjects cultivated by conventional methods and experimental protocols
[0045] All the test subjects were in stage IV and had no other treatment, and their legal representatives were informed and agreed to receive this immunotherapy.
[0046] The cells obtained from the two schemes were reinfused intravenously to 11 test subjects, and the peripheral blood CD3 was detected before and after treatment (2 months after the end of treatment). + , CD8 + , CD56 + Variety.
[0047] All data are expressed as mean and standard deviation, t test, and SPSS18.0 software package is used for analysis.
[0048] After 2 months of treatment by conventional methods, all patients with CD3 + , CD8 + and CD56 + All were significantly higher than before treatment (P<0.001) (Table 3).
[0049] Table 3 Statistical analysis results of immune indexes of T lymphocyte subsets before treatment and after 2 months of treatmen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com