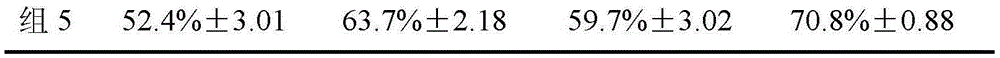Multi-cell immune preparation for treating tumors and preparation method of multi-cell immune preparation
An immune preparation, multi-cell technology, applied in anti-tumor drugs, animal cells, vertebrate cells, etc., can solve problems such as the inability to guarantee the safety of reinfusion and the limitations of tumor treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
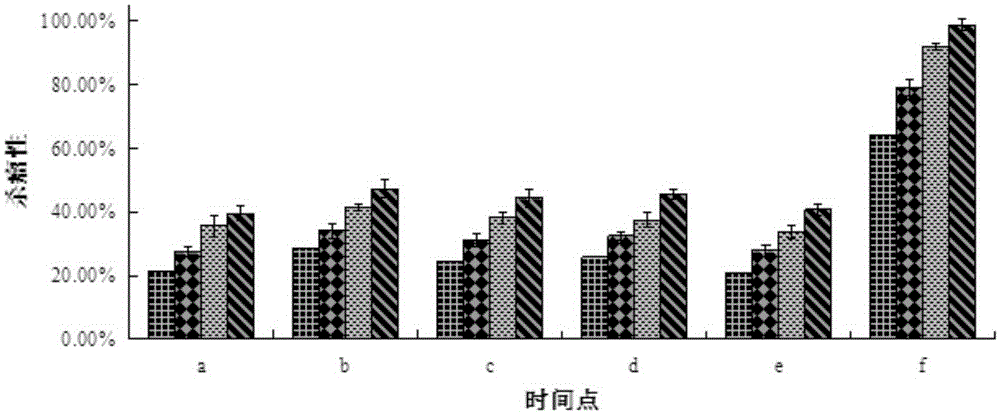
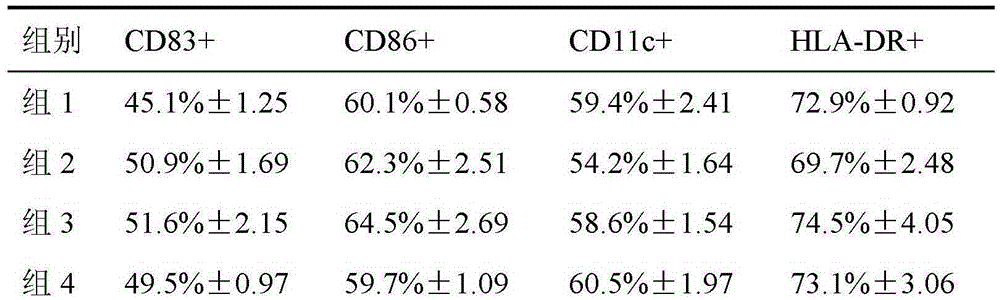
Image
Examples
specific Embodiment approach 1
[0060] Specific embodiment 1: The multicellular immune preparation for treating tumors in this embodiment includes immune cells, human serum albumin and compound electrolytes, and the immune cells include DC cells, CIK cells, NK cells, CD3AK cells and γδT cells; the immune cells The concentration is 1×10 7 cells / ml~5×10 7 cells / ml, the concentration of human serum albumin is 1-2g / 100ml.
specific Embodiment approach 2
[0061] Specific embodiment two: the difference between this embodiment and specific embodiment one is that the concentration of immune cells is 2×10 7 cells / ml~4×10 7 cells / ml. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0062] Specific embodiment three: The preparation method of the multicellular immune preparation for treating tumors in this embodiment is carried out according to the following steps:
[0063] 1. Preparation of autologous plasma:
[0064] ①Collect adult peripheral blood, the anticoagulant is sodium citrate, transfer the collected blood to a centrifuge tube, and centrifuge to collect plasma;
[0065] ② Inactivate the collected plasma and bathe in water at 56°C for 30-50 minutes. After the water bath is completed, centrifuge to collect the supernatant, which is autologous plasma, ready for use;
[0066] 2. Isolation of PBMC from peripheral blood mononuclear cells:
[0067] ① Divide the Ficoll solution into centrifuge tubes and wait for use. Mix adult peripheral blood cells and compound electrolytes according to the volume ratio (1-2): 1 to obtain a diluted cell suspension. Add the diluted cell suspension To the centrifuge tube filled with Ficoll solution, the volume ratio of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com