Method for differentiating human pluripotent stem cells into natural killer cells and application
A technology of natural killer cells and pluripotent stem cells, applied in the field of stem cell biology, can solve the problems of long differentiation cycle, low differentiation efficiency and purity, dependence on feeder cells, etc., and achieve short cycle, low cost and clear composition Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] This embodiment discloses a method for preparing natural killer cells, comprising the following steps:
[0137] S1: Formation of embryoid bodies;
[0138] S2: Differentiation of embryoid bodies to hematopoietic progenitor cells;
[0139] S3: differentiation of hematopoietic progenitor cells into NK cells;
[0140] S4: Maturation and expansion of NK cells.
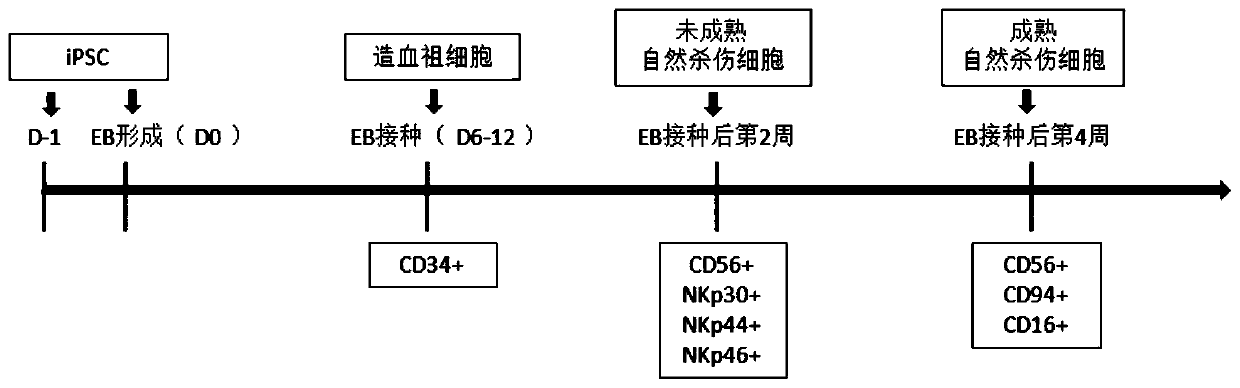
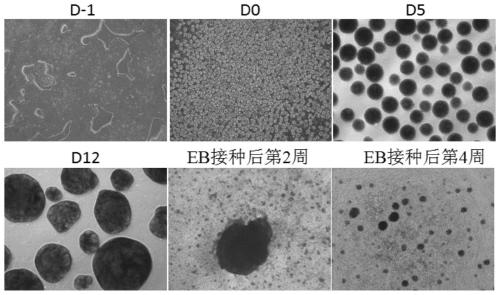
[0141] Specifically, the flow chart is as figure 1 As shown, the steps are as follows:
[0142] (1) Day-1~Day 0: Formation of embryoid body (EB)
[0143] 1) Culture of human pluripotent stem cells (iPSCs)
[0144] The human iPSCs used in the experiment have undergone strict pluripotency verification (expressing various pluripotency markers, and can form teratomas including inner, middle and outer germ layers in immunodeficient mice). iPSCs are normally cultured in iPSC maintenance medium, the medium used is E8 or TeSR or other similar medium.
[0145] The human iPSC is prepared by the method disclosed in the ...
Embodiment 2
[0193] This example studies the effects of different EB culture methods on the EB differentiation rate. In the experimental group, the culture method of 3D suspension culture to form embryoid bodies was adopted throughout the induction and differentiation process, that is, the iPSCs were directly formed into smaller volume EBs on a 3D shaker, and then replaced with different special differentiation media at different differentiation stages ( Concrete steps refer to embodiment 1). During the culture process, the volume of EB gradually increased, and the cells continued to proliferate. Compared with the 2D differentiation scheme (see patent CN107429230A), the 3D differentiation condition greatly saves the culture space and culture volume, and the number of cells obtained under the same culture system is significantly increased, which is conducive to the scale-up of hPSCs to multipotential blood precursor cells Production. Compared with the differentiation scheme of Spin EB (US...
Embodiment 3
[0195] The present invention finds that in 3D induction culture, the use of small molecule reagents, cytokines and cell matrix can replace serum and trophoblast cells, and accelerate the differentiation process and improve the differentiation efficiency, which is beneficial to the production of subsequent clinical-grade cell preparations.
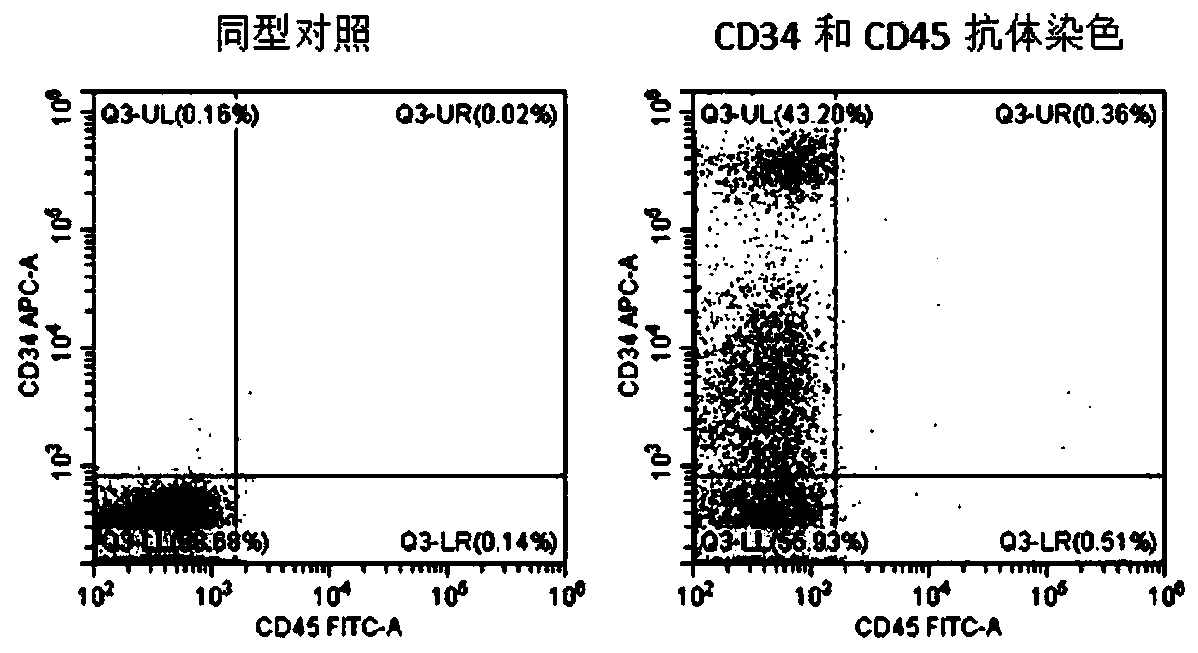
[0196] In our differentiation method, in addition to using 3D to improve differentiation efficiency, the combined use of small molecule reagents, cytokines, and cell substrates not only avoids the use of serum and trophoblast cells, but also further increases the proportion of blood precursor cells. CHIR99021 was selected as the GSK3β inhibitor, BMP4 was used as the BMP signaling pathway activator, SB431542 was used as the Nodal inhibitor, and DLL4-Fc recombinant protein was used as the Notch signaling pathway activator in the cell matrix. On days 6-12, the ratio of blood progenitor cells (CD34+) obtained can be as high as 20-80%, which is h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com