In-vitro pure culture method for natural killer cells
A technology of natural killer cells and pure culture, applied in the field of pure culture of natural killer cells in vitro, can solve problems such as high safety risks, and achieve the effects of high amplification multiples, high safety, and clear medium components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
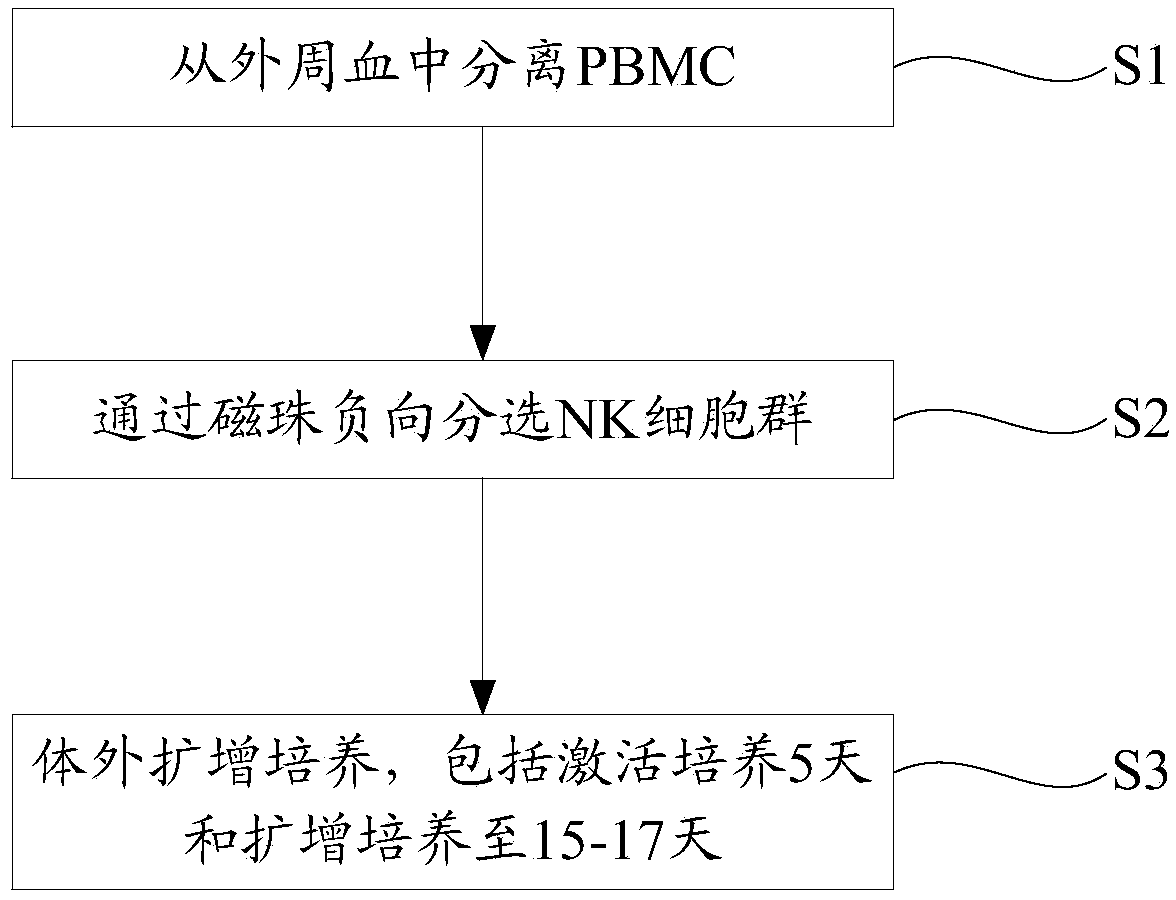
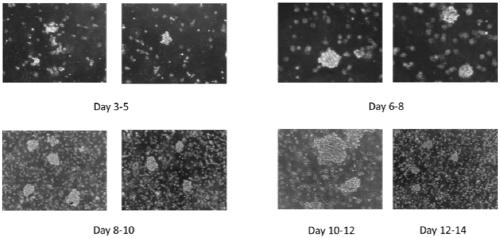
[0050] Please combine figure 1 , the present embodiment provides a method for in vitro pure culture of natural killer cells, comprising the following steps:
[0051] S1, collecting peripheral blood and separating PBMC:
[0052] 50 mL of peripheral blood was drawn from a venous vessel and placed in a sodium heparin anticoagulant blood collection tube. Subsequently, the peripheral blood was transferred to a 50mL centrifuge tube and centrifuged at 580g for 30 minutes. After centrifugation, the liquid in the centrifuge tube was divided into two layers, the upper layer was plasma, and the lower layer was cell layer.
[0053] Carefully draw the upper layer of plasma, put it in a 50mL centrifuge tube, inactivate it in a dry bath at 56°C for 30 minutes, then centrifuge it at 800g for 20 minutes, remove the supernatant, and store in a refrigerator at -20°C.
[0054] Resuspend the lower cell layer with about 25mL PBS buffer to obtain a uniform cell mixture. Take two new 50mL centrifu...
Embodiment 2
[0064] The influence of embodiment 2 culture medium composition on NK cell expansion efficiency
[0065] In this example, the influence of the composition of the culture medium on the expansion efficiency of NK cells was explored by adopting the method of in vitro pure culture of natural killer cells in Example 1. Wherein, the composition of control medium is shown in Table 1 below:
[0066] Table 1 Composition table of control culture medium
[0067]
[0068]
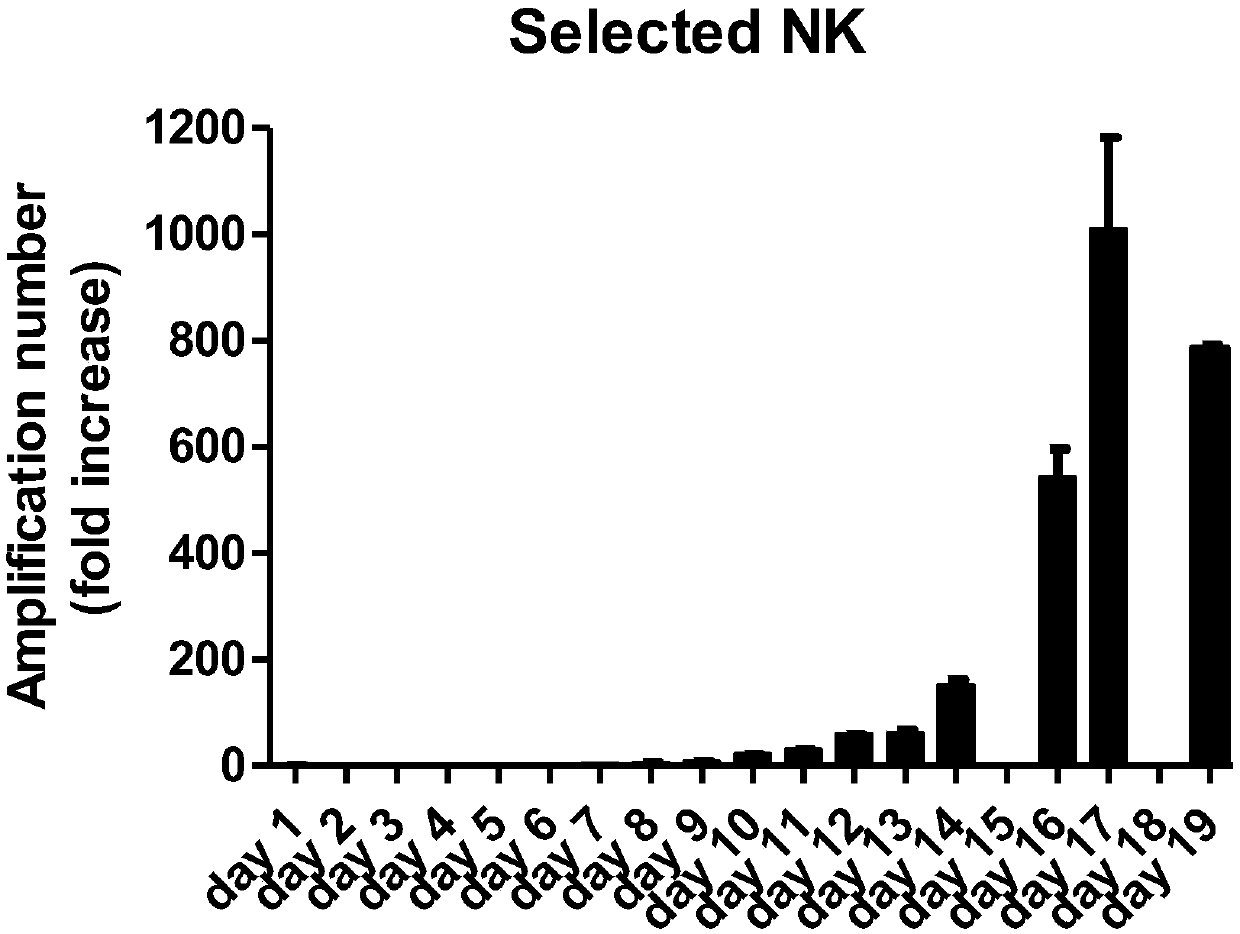
[0069] In this example, the cells under different culture conditions are counted, and the proliferation rate is compared with the statistical basis of "the concentration of OK432 is 0 μg / mL, and the concentration of IL-2 is 300 IU", and the statistical results are shown in Figure 4 .
[0070] Depend on Figure 4 It can be seen that under the same concentration of OK432, the effect of higher concentration of IL-2 on the proliferation of NK cells is obvious. Under the condition that the culture medium contains...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com