Mannose-containing solution for lyophilization, transfection and/or injection of nucleic acids
a technology of lyophilization and mannose, which is applied in the direction of antibody medical ingredients, drug compositions, immunological disorders, etc., can solve the problems of reducing the transfection efficiency, reducing the biological activity reducing the expression of the encoded protein in vitro, so as to increase the transfection efficiency increase the in vivo expression of the encoded protein, and improve the stability of the nucleic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Plasmids
[0733]For the present examples DNA sequences encoding Photinus pyralis luciferase as well as DNA sequences encoding Ovalbumin were prepared and used for subsequent in vitro transcription reactions and expression studies.
[0734]According to a first preparation, the DNA sequence corresponding to pCV19-Ppluc(GC)-muag-A70-C30 was prepared, which encodes the Photinus pyralis luciferase coding sequence. The constructs were prepared by modifying the wild type Photinus pyralis luciferase encoding DNA sequence by introducing a GC-optimized sequence for a better codon usage and stabilization, stabilizing sequences derived from alpha-globin-3′-UTR (muag (mutated alpha-globin-3′-UTR)), a stretch of 70× adenosine at the 3′-terminal end (poly-A-tail) and a stretch of 30× cytosine at the 3′-terminal end (poly-C-tail), corresponding to SEQ ID NO: 1 (see FIG. 5). The sequence of the final DNA construct had a length of 1857 nucleotides. The corresponding mRNA sequence was termed...
example 2
In Vitro Transcription
[0741]The respective DNA plasmids prepared according to Example 1 were transcribed in vitro using T7-Polymerase (T7-Opti mRNA Kit, CureVac, Tibingen, Germany) following the manufactures instructions. Subsequently the mRNA was purified using PureMessenger® (CureVac, Tubingen, Germany).
example 3
Lyophylisation
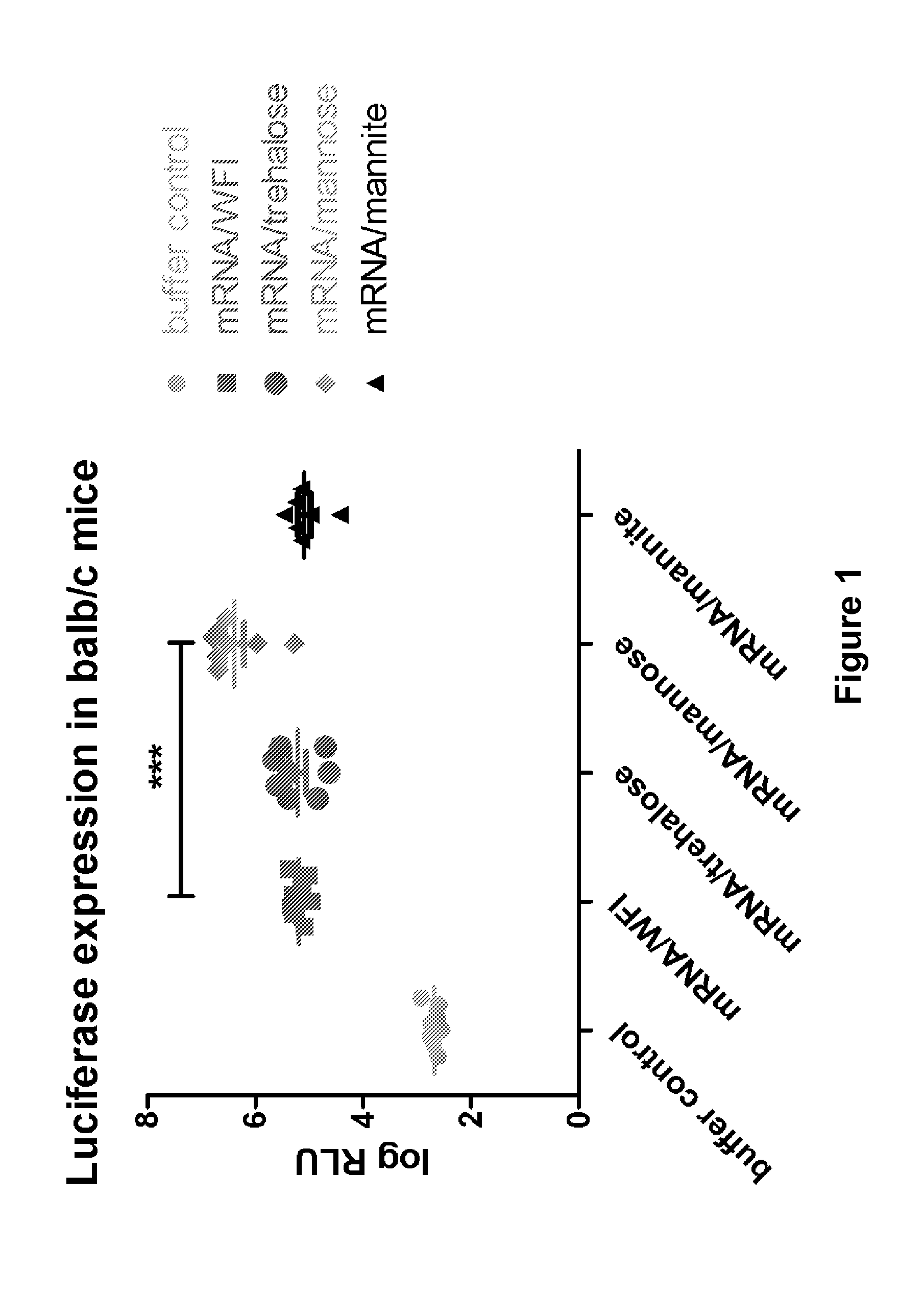
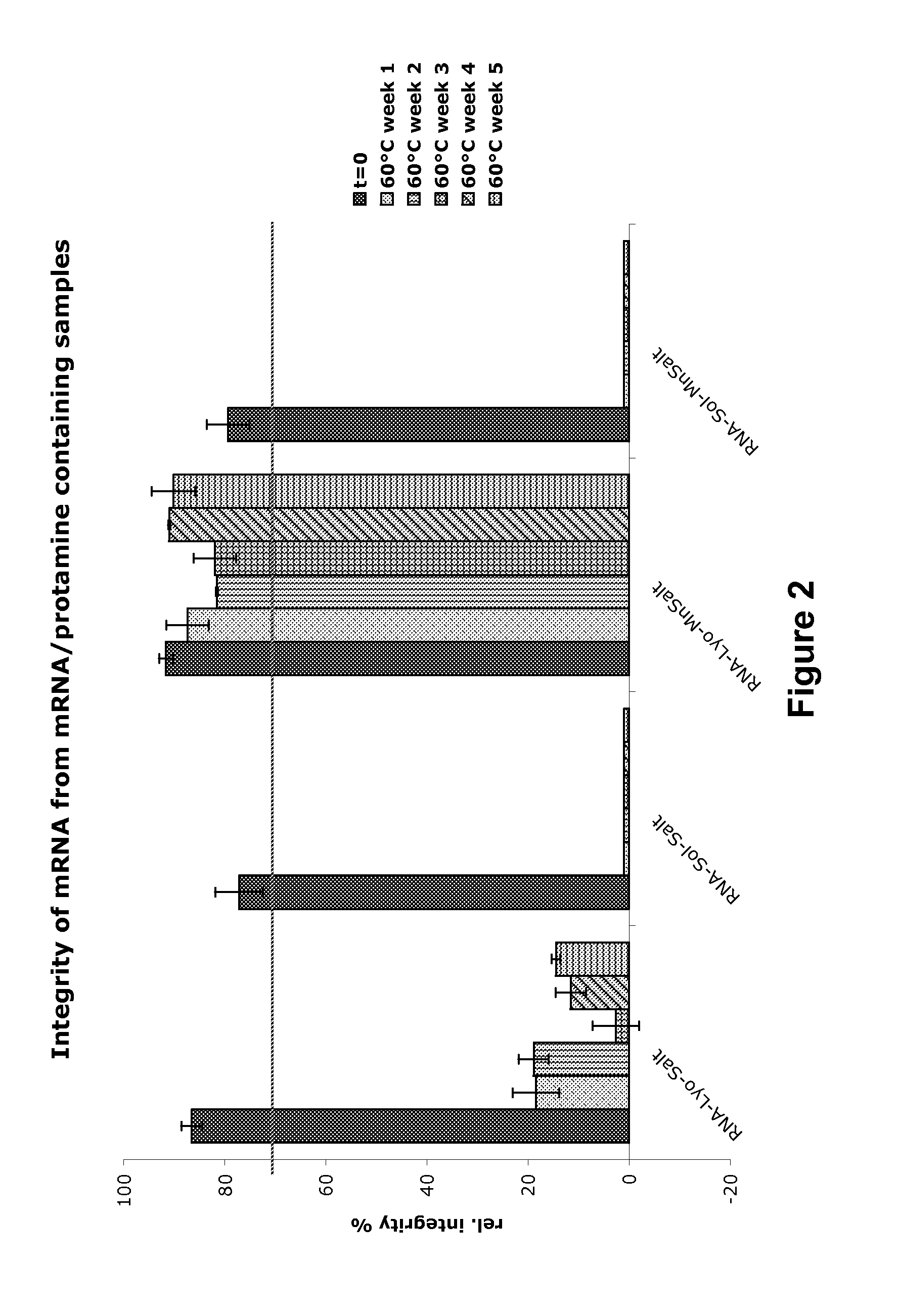
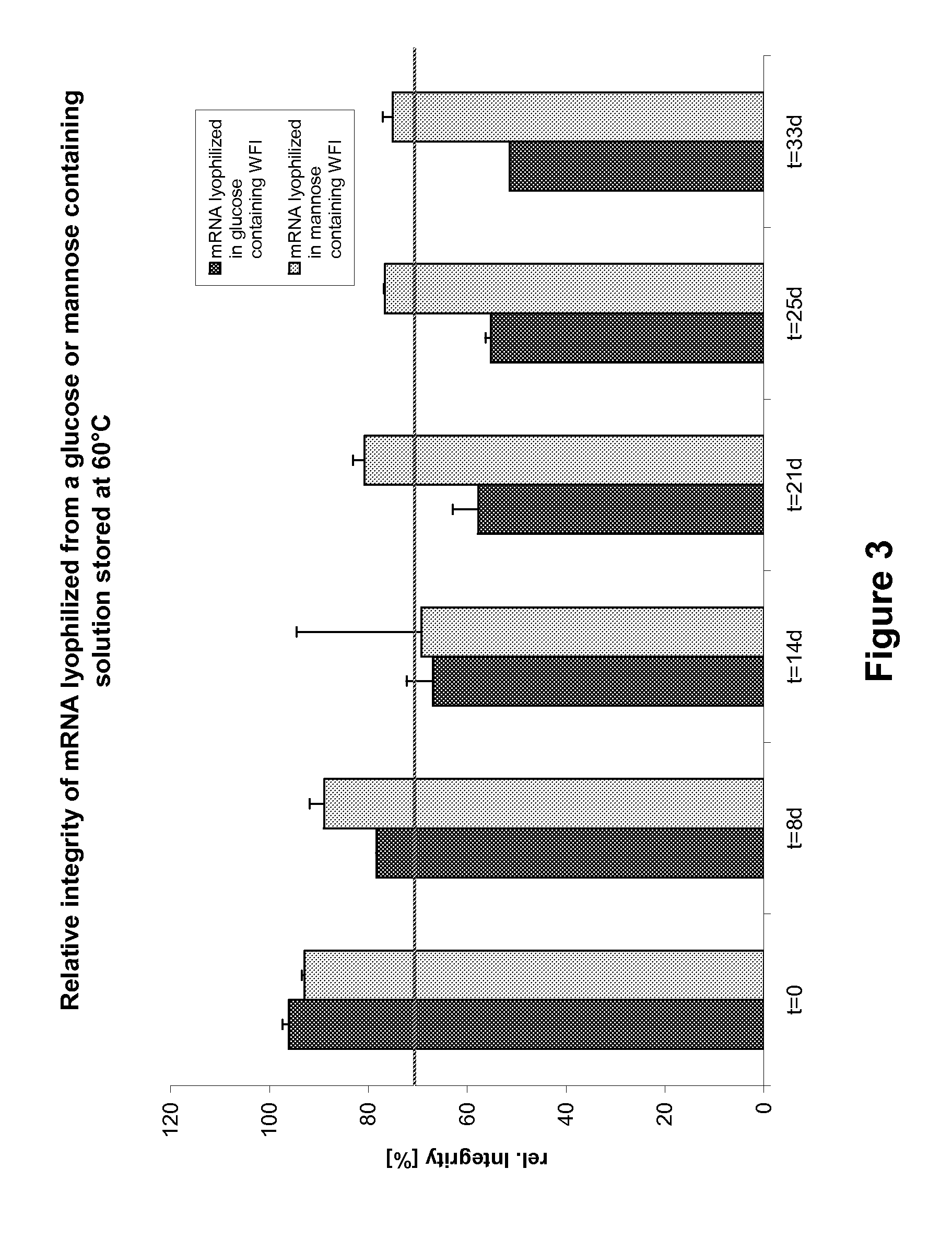
[0742]The PureMessenger® purified and precipitated mRNA obtained according to Examples 1 and 2 coding for Photinus pyralis luciferase (Luc mRNA) (SEQ ID NO: 1) or Ovalbumin (SEQ ID NO: 2) were prepared for transfection and expression tests.
[0743]The PureMessenger® purified and precipitated mRNA obtained according to Examples 1 and 2 coding for Photinus pyralis luciferase (Luc mRNA) (SEQ ID NO: 1) or Ovalbumin (SEQ ID NO: 2) was dissolved in water for injection (WFI) to 5 g / l. Subsequently the mRNA was diluted with WFI (water for injection) or salt solution (see FIG. 2), with addition of glucose, trehalose, mannite or mannose. Aliquots of these solutions were lyophilized (Controls were frozen in liquid nitrogen or kept in solution). The locked cups were stored for the indicated time at 60° C. The resuspension was conducted with WFI.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com