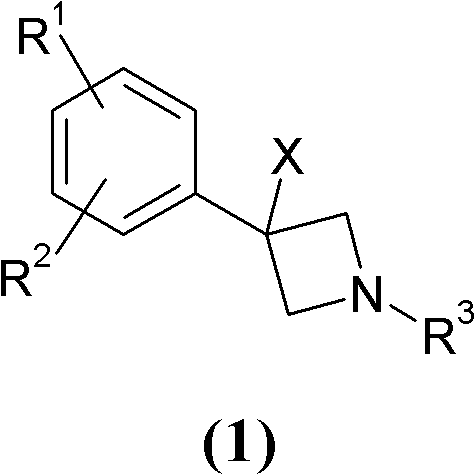
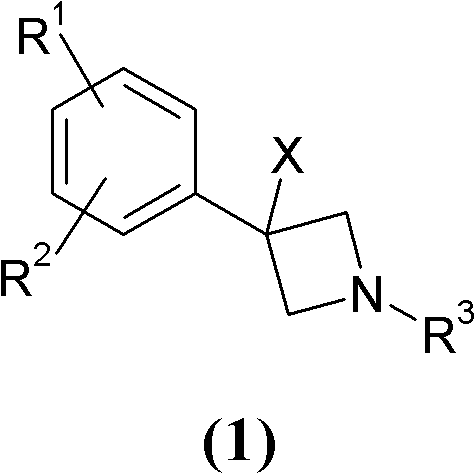
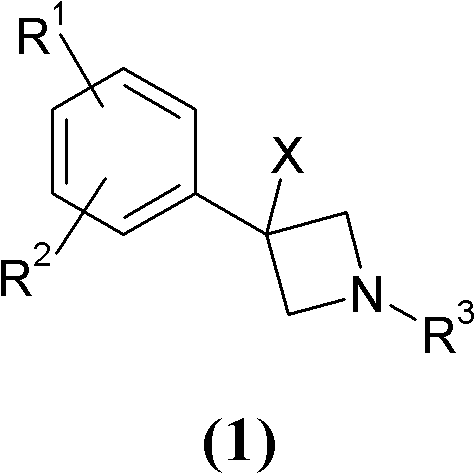
Novel 3-phenyl-azetidine derivatives useful as modulators of cortical catecholaminergic neurotransmission
A technology of azetidine and azetidine, applied in the field of new 3-phenyl-azetidine derivatives, can solve the problem of unreported 3-phenyl-azetidine derivatives, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] 3-(2,3-Difluorophenyl)-1-ethylazetidin-3-ol
[0128] 1,3-Dichloro-2-(2,3-difluorophenyl)propan-2-ol (6.0 g, 24.89 mmol) was dissolved in acetonitrile (90 ml), transferred to 6 different microwave vials, Each vial contained an equal amount (1.0 g, 2.49 mmol). Sodium bicarbonate (1.04 g, 12.44 mmol (total 6.27 g, 74.67 mmol)) was added to each tube and the tube was sealed. Ethylamine in tetrahydrofuran (2.0M, 2.07ml, 4.15mmol) was added through the septum (total 12.44ml, 24.89mmol), and the mixture was heated under microwave irradiation at 120°C for 30min. Ethylamine in tetrahydrofuran (2.0M, 1.03ml, 2.07mmol (a total of 6.22ml, 12.44mmol), the mixture was heated under microwave irradiation at 120°C for 30min. A solution of water (100ml) and tert-butyl methyl ether (100ml) was added thereto, and the organic phase was collected. Using tert-butyl methyl ether (100ml) to extract the aqueous phase and dry the combined organic phase (Na 2 SO 4 ) and evaporate. The crude p...
Embodiment 2
[0130] 3-(3,4-Difluorophenyl)-1-ethylazetidin-3-ol
[0131] 3-(3,4-difluorophenyl)azetidin-3-ol (0.3g, 1.62mmol) triethylamine (0.68ml, 4.87mmol) and iodoethane (0.19ml, 2.44mmol ) in tetrahydrofuran (15ml) was stirred at ambient temperature for 15 hours. The solvent was evaporated, aqueous hydrochloric acid (50 mL. 10%) was added and the mixture was washed with tert-butyl methyl ether (2 x 50 mL). The aqueous phase was made basic by addition of aqueous sodium hydroxide (5M) and extracted with tert-butyl methyl ether (2 x 50ml). The combined organic phases were dried (Na 2 SO 4 ), evaporated under reduced pressure to give the crude product (0.26 g). Purification by flash column chromatography (ethyl acetate / methanol 1:1) on silica gel afforded 0.2 g (58%) of the title compound. Amine conversion to fumarate, recrystallization from methanol / ether: M.p. 177-179°C. MS m / z (relative intensity, 70 eV) 213 (M+, 1), 141 (86), 127 (45), 114 (58), 58 (bp).
Embodiment 3
[0133] 3-(3,5-Difluorophenyl)-1-ethylazetidin-3-ol
[0134] Dissolve 1,3-dichloro-2-(3,5-difluorophenyl)propan-2-ol (1.0g, 4.15mmol) in acetonitrile (10ml), add sodium bicarbonate (1.39g, 16.59mmol and ethylamine in water (70%, 0.33ml, 4.15mmol), the mixture was refluxed for 10 hours. Water (50ml) and tert-butyl methyl ether (50ml) were added, and the organic phase was collected. The aqueous phase was extracted with tert-butyl methyl ether (50ml, The combined organic phases were extracted with aqueous hydrochloric acid (10%, 50 ml). The aqueous phase was basified, extracted with tert-butyl methyl ether (2×50 ml), dried (Na 2 SO 4 ) and evaporated to give 0.35 g of crude product. Purification by flash column chromatography (ethyl acetate / MeOH 1:0→1:1) on silica gel afforded the title compound (0.2 g, 23%). Amine conversion to fumarate, recrystallization from ethanol / diethyl ether / diisopropyl ether: M.p. 140-141°C. MS m / z (relative intensity, 70 eV) 213 (M+, 1), 156 (36), 14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com