New disubstituted phenylpyrrolidines as modulators of cortical catecholaminergic neurotransmission
A technology of pyrrolidine and difluorophenyl, applied in the field of novel disubstituted phenylpyrrolidine and these compounds, can solve the problem of no norepinephrine and dopamine neurotransmission, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
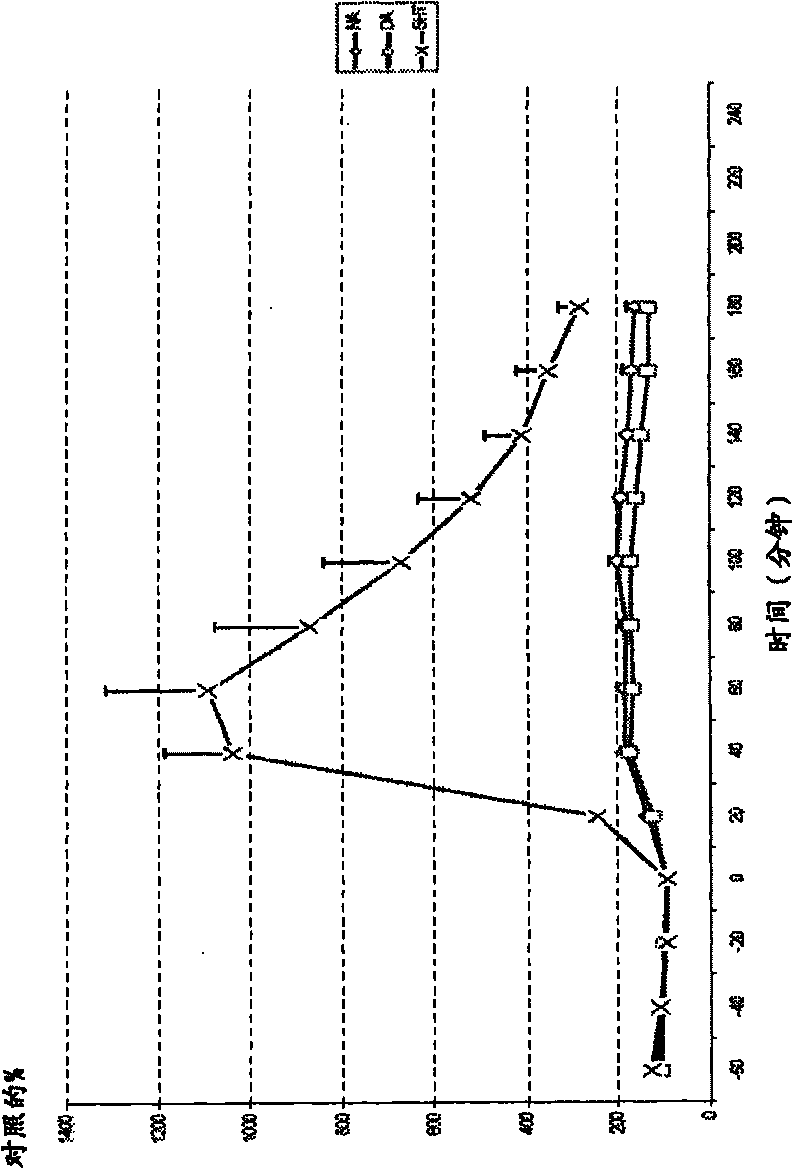
[0187] figure 1 .Example 1, 50 μmol / kg subcutaneous striatal amine
[0188] Example 1 (subcutaneous injection) was injected at time point 0. exist figure 1 Values stated in represent percentage of control relative to baseline value. Microdialysis was performed in conscious and freely moving rats. Dopamine = DA; Norepinephrine = NA; Serotonin = 5-HT; Error bars = SEM
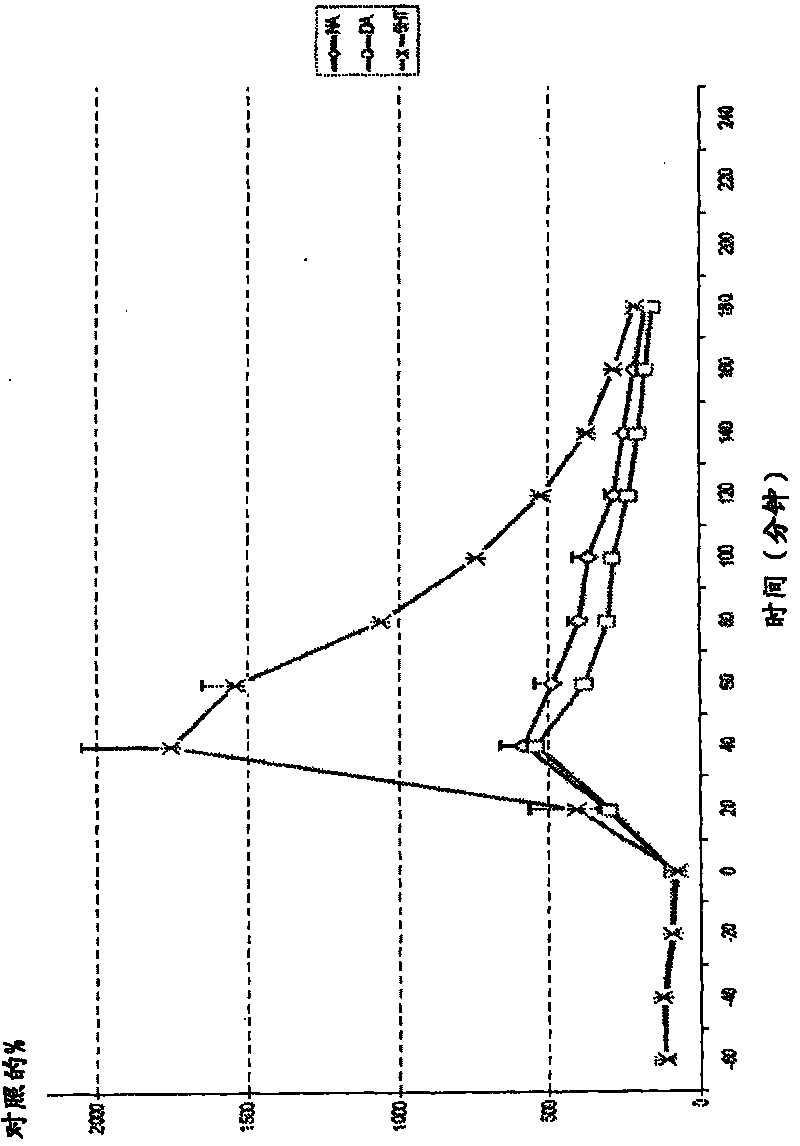
[0189] figure 2 .Example 1, 50 μmol / kg subcutaneously p.f. cortical amine
[0190] Example 1 (subcutaneous injection) was injected at time point 0. exist figure 2 Values stated in represent percentage of control relative to baseline value. Microdialysis was performed in conscious and freely moving rats. Dopamine = DA; Norepinephrine = NA; Serotonin = 5-HT; Error bars = SEM
Embodiment 2
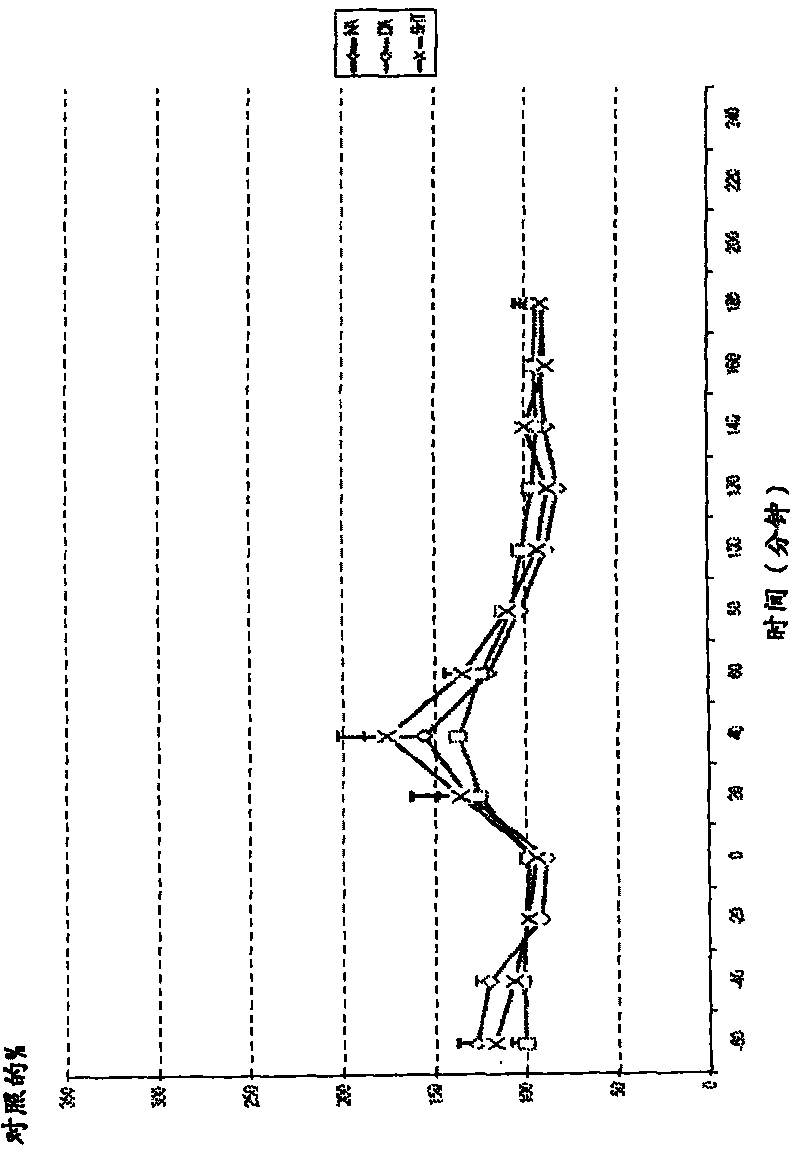
[0191] image 3 .Example 2, 50 μmol / kg subcutaneous striatal amine
[0192] Example 2 was injected at time point 0 (subcutaneous injection). exist image 3 Values stated in represent percentage of control relative to baseline value. Microdialysis was performed in conscious and freely moving rats. Dopamine = DA; Norepinephrine = NA; Serotonin = 5-HT; Error bars = SEM
[0193] Figure 4 .Example 2, 50 μmol / kg subcutaneously p.f. cortical amine
[0194] Example 2 was injected at time point 0 (subcutaneous injection). exist Figure 4 Values stated in represent percentage of control relative to baseline value. Microdialysis was performed in conscious and freely moving rats. Dopamine = DA; Norepinephrine = NA; Serotonin = 5-HT; Error bars = SEM
[0195] Figure 5 : Example 3, 50 μmol / kg subcutaneous striatal amine
[0196] Example 3 was injected at time point 0 (subcutaneous injection). exist Figure 5 Values stated in represent percentage of control relati...
Embodiment 5
[0203] Figure 9 .Example 5, 50 μmol / kg subcutaneous striatal amine
[0204] Example 5 was injected at time point 0 (subcutaneous injection). exist Figure 9 Values stated in represent percentage of control relative to baseline value. Microdialysis was performed in conscious and freely moving rats. Dopamine = DA; Norepinephrine = NA; Serotonin = 5-HT; Error bars = SEM
[0205] Figure 10 .Example 5, 50 μmol / kg subcutaneously p.f. cortical amine
[0206] Example 3 was injected at time point 0 (subcutaneous injection). exist Figure 10 Values stated in represent percentage of control relative to baseline value. Microdialysis was performed in conscious and freely moving rats. Dopamine = DA; Norepinephrine = NA; Serotonin = 5-HT; Error bars = SEM
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com