Enhanced delivery of viral particles to the striatum and cortex
a technology of viral particles and striatum, which is applied in the direction of viruses/bacteriophages, genetic material ingredients, drug compositions, etc., can solve the problems of affecting the effectiveness of aav vector distribution, affecting the effective treatment of neurologic disorders, and simple injections, etc., to increase the safety and efficacy of ced a reflux-resistant cannula, improve the safety and efficacy, and improve the safety. the effect of the
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
d GFP Expression after Intrastriatal AAV1 Vector Delivery
[0201]The ability of AAV1 to efficiently target both striatal and cortical structures in the Rhesus monkey brain when delivered via convection-enhanced delivery (CED) was evaluated. AAV vectors containing GFP cDNA under the control of cytomegalovirus enhancer / chicken beta-actin (CBA) promoter were infused into the caudate and putamen of 9 adult male Rhesus monkeys using CED (see, e.g., Bankiewicz et al., (2000) Exp. Neurol. 164:2-14 and WO 2010 / 088560).
[0202]Methods
Surgical Delivery
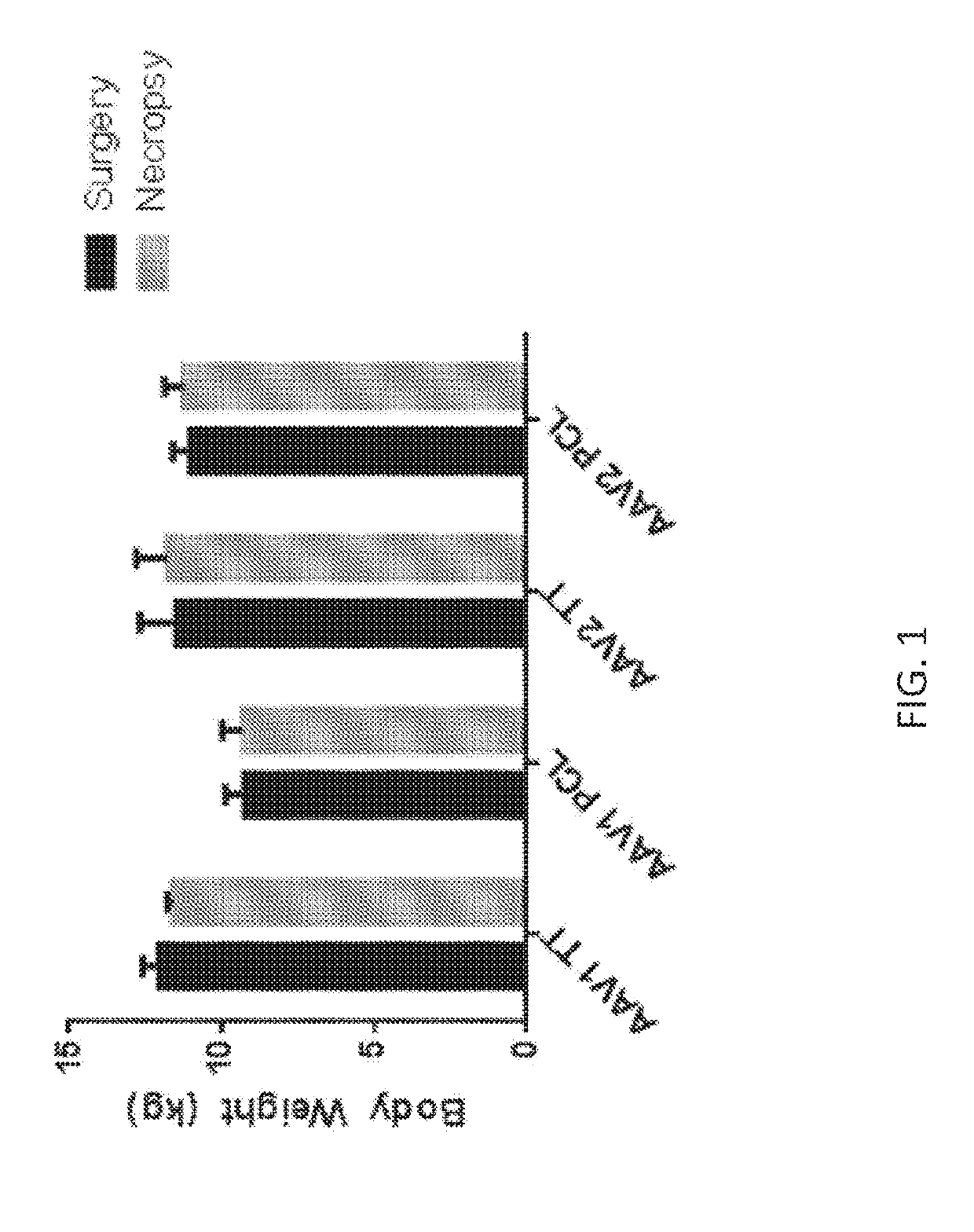
[0203]Nine adult male Rhesus macaques (Macaca mulatta; 8.9-11.9 kg) were included in this study. All animals received an infusion of AAV vector bilaterally into caudate nucleus and putamen by means of MRI-guided CED (Richardson, R. M. et al. (2011) Neurosurgery 69:154-163; Richardson, R. M. et al. (2011) Stereotact. Funct. Neurosurg. 89:141-151; Richardson, R. M. et al. (2011)Mol. Ther. 19:1048-1057). Immediately prior to surgery, animals were anest...
example 2
d GFP Expression after Intrastriatal AAV2 Vector Delivery
[0238]The ability of AAV2 to efficiently target both striatal and cortical structures in the Rhesus monkey brain when delivered via convection-enhanced delivery (CED) was evaluated. AAV vectors containing GFP cDNA under the control of cytomegalovirus enhancer / chicken beta-actin (CBA) promoter were infused into the caudate and putamen of 8 adult Rhesus monkeys using CED according to the methods described in Example 1 above.
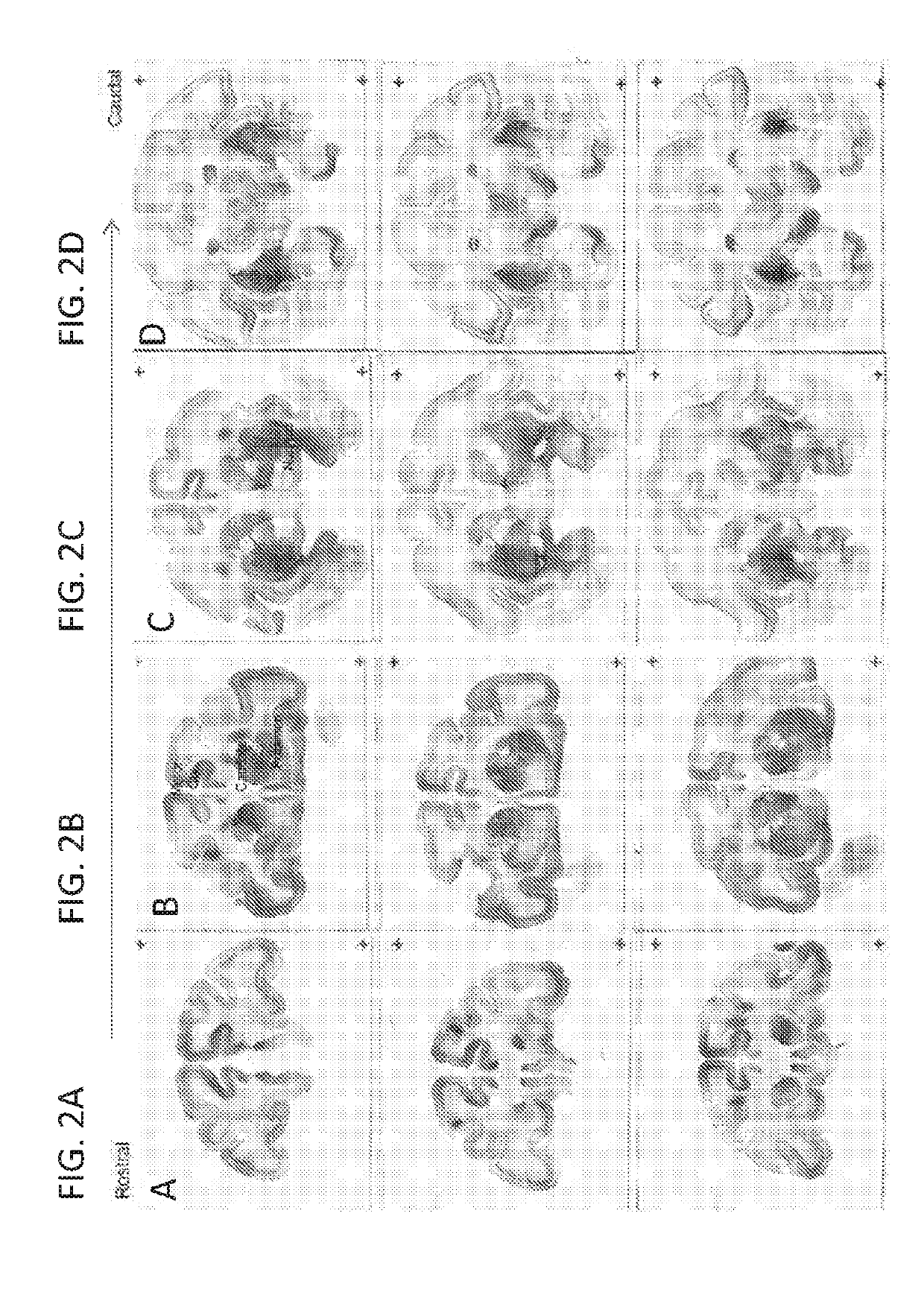
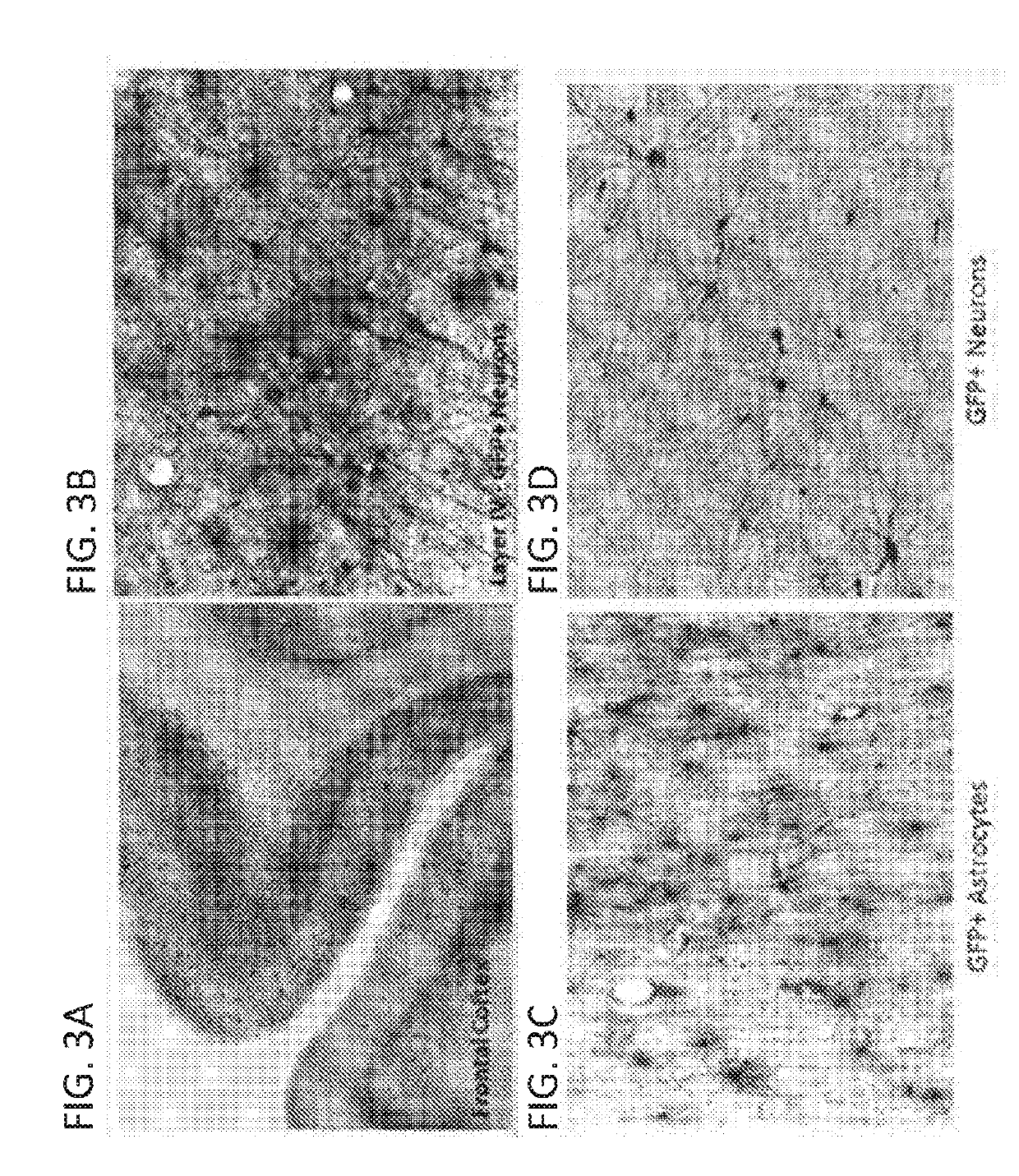
[0239]Infusion of AAV2 into the striatum by CED resulted in GFP expression in the injected regions (caudate and putamen) (FIG. 4C), substantia nigra (FIG. 4D), and a large number of cortical regions of the Rhesus monkey brain (FIGS. 4A-D). Expression of GFP in the striatum of AAV2 injected animals appeared slightly more restricted and localized when compared to striatal coverage with AAV1 vectors. The expression of GFP within the NHP striatum was comprehensive but relatively contained within the gray matter b...
example 3
lity of GFP Expression after Intrastriatal AAV1 and AAV2 Vectors Made by Triple Transfection or Producer Cell Line Process
[0240]To date the majority of preclinical studies utilize AAV vectors made by Triple Transfection followed by purification using cesium chloride gradients or column chromatography. Thus, to evaluate the impact of vector production on biodistribution in vivo, two methods of vector production Triple Transfection (TT) or Producer Cell line (PCL), were compared. AAV1 and AAV2 vectors generated by these two different manufacturing platforms were administered via CED, and their distribution within the Rhesus monkey brain was compared.
[0241]Infusion of AAV1-GFP vectors made by triple transfection yielded equivalent GFP distribution and coverage when compared to AAV1-GFP vectors made by the producer cell line process. GFP distribution was comparable between AAV1-GFP (TT) (FIGS. 5C&D) and AAV1-GFP (PCL) (FIGS. 5A&B) vectors 30 days following injection into the striatum of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com