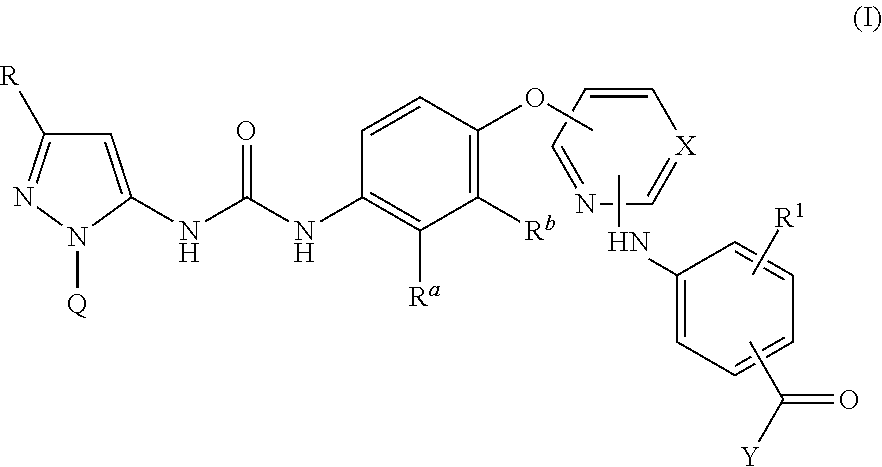
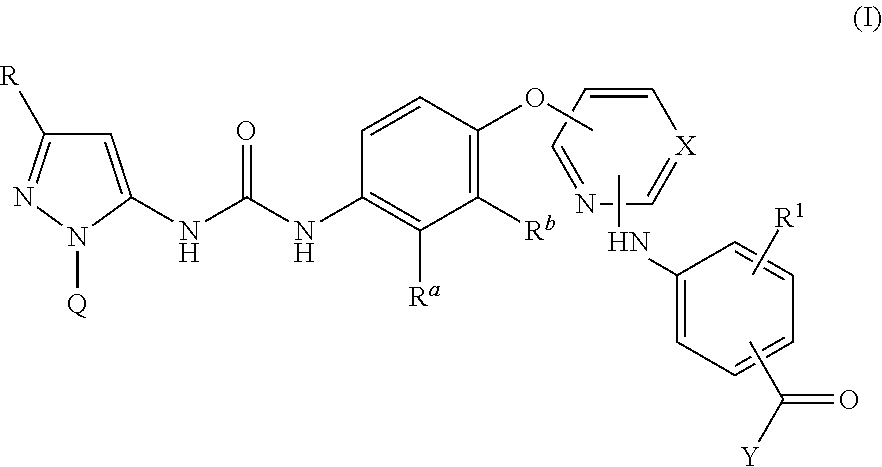
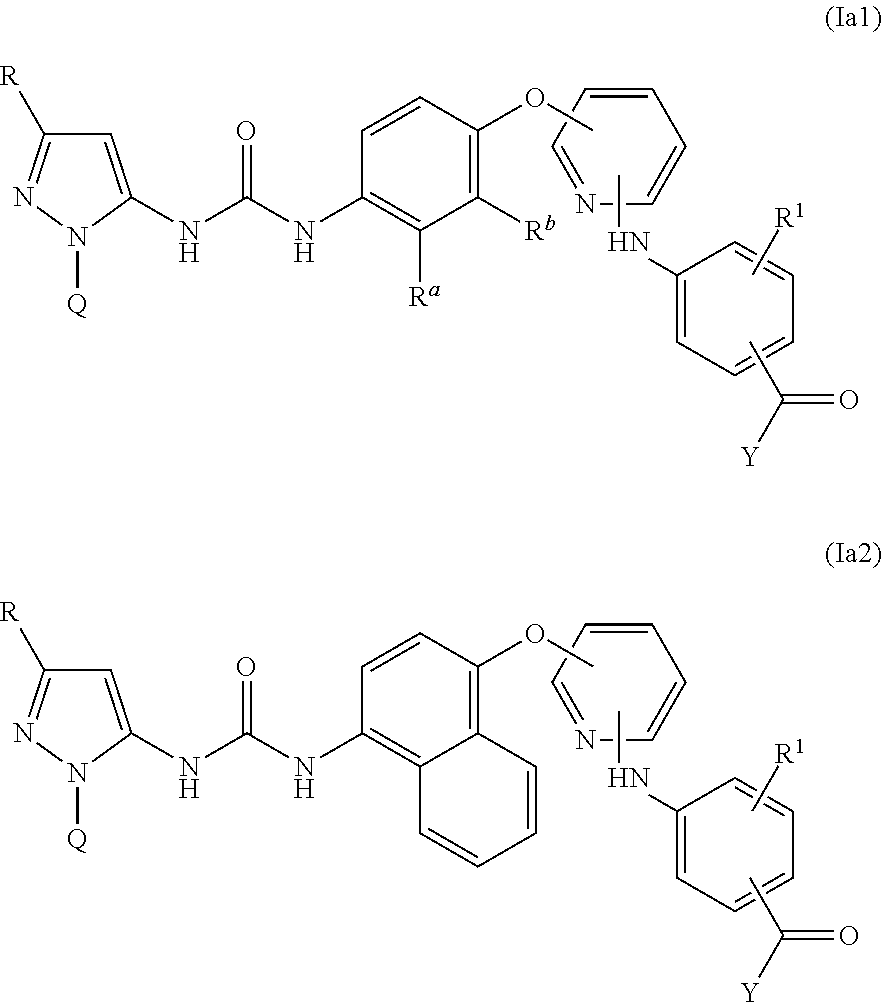
Kinase inhibitors
a technology of kinase inhibitors and inhibitors, applied in the field of kinase inhibitors, can solve the problems of multiple off-target effects, high toxicity, and discontinuation of development of a substantial number of inhibitors, and achieve the effect of enhancing the therapeutic profile and low affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
3-((4-((4-(3-(3-(tert-Butyl)-1-(p-tolyl)-1H-pyrazol-5-yl)ureido)naphthalen-1-yl)oxy)pyridin-2-yl)amino)-5-methoxy-N-(2-morpholinoethyl)benzamide
[0680]
[0681]To a solution of Intermediate A8 (74 mg, 0.32 mmol) in DCM (1.0 mL) was added CDI (54 mg, 0.34 mmol) and the reaction mixture kept at 40° C. for 2 hr. An aliquot of this solution (310 μL, 0.099 mmol), containing the pre-formed pyrazole CDI adduct, was added to a solution of Intermediate B1 (50 mg, 0.078 mmol) in THF (1.0 mL) at RT and the resulting mixture maintained at this temperature for 18 hr. A second aliquot of the pyrazole CDI adduct (160 μL, 0.05 mmol) was then added and after 3 hr at RT the reaction mixture was partitioned between EtOAc (50 mL) and saturated aq NaHCO3 (50 mL). The organic phase was separated and was washed sequentially with saturated aq NaHCO3 (2×50 mL), water (2×50 mL) and brine (2×50 mL) and then dried and evaporated in vacuo. The residue was purified by preparative HPLC to afford the title compound, E...
example 2
3-((4-((4-(3-(3-(tert-Butyl)-1-(p-tolyl)-1H-pyrazol-5-yl)ureido)naphthalen-1-yl)oxy)pyrimidin-2-yl)amino)benzamide
[0682]
[0683]To a suspension of Intermediate F1 (50 mg, 0.080 mmol) in THF (1.5 mL) was added DIPEA (28 μL, 0.16 mmol) and HATU (36 mg, 0.096 mmol) and after 10 min at RT the reaction mixture was treated with NH4Cl (4.7 mg, 0.088 mmol). The resulting mixture was maintained at RT for 18 hr and was then partitioned between saturated aq NaHCO3 (3.0 mL) and EtOAc (3.0 mL). The organic phase was separated and was washed with hydrochloric acid (1.0 M, 3.0 mL) and with brine (3.0 mL) and then dried and evaporated in vacuo. The residue was purified by flash column chromatography (SiO2, 4.0 g, MeOH in EtOAc, 0-100%, gradient elution) to afford the title compound, Example 2, as a white solid (8 mg, 16%); Rt 2.30 min (Method 2 acidic); m / z 627 (M+H)+ (ES+); m / z 625 (M−H)− (ES−); 1H NMR δ: 1.29 (9H, s), 2.40 (3H, s), 6.42 (1H, s), 6.55 (1H, d), 6.99 (1H, m), 7.23 (1H, br s), 7.29 (1H...
example 3
3-((4-((4-(3-(3-(tert-Butyl)-1-(p-tolyl)-1H-pyrazol-5-yl)ureido)naphthalen-1-yl)oxy)pyrimidin-2-yl)amino)-N-(2-morpholinoethyl)benzamide
[0684]
[0685]To a suspension of Intermediate F1 (680 mg, 1.08 mmol) in DCM (10 mL) at 0° C. was added oxalyl chloride (110 μL, 1.30 mmol) and DMF (1 drop) and the resulting red mixture maintained at 0° C. for 20 min and then warmed to RT. After 1 hr a second aliquot of oxalyl chloride (110 μL, 1.30 mmol) was added and the resulting mixture kept at RT for 2 hr and then evaporated in vacuo to afford a red solid (800 mg). This material was used in the subsequent amide coupling without purification or characterization. To a suspension of a portion of the solid obtained above (60 mg, 0.080 mmol) in DCM (1.5 mL) was added DIPEA (32 μL, 0.19 mmol) and 2-morpholinoethanamine (13 μL, 0.10 mmol) and the reaction mixture maintained at RT for 3 hr. The resulting mixture was washed sequentially with saturated aq. NaHCO3 (5.0 mL), water (5.0 mL) and with brine (5....
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com