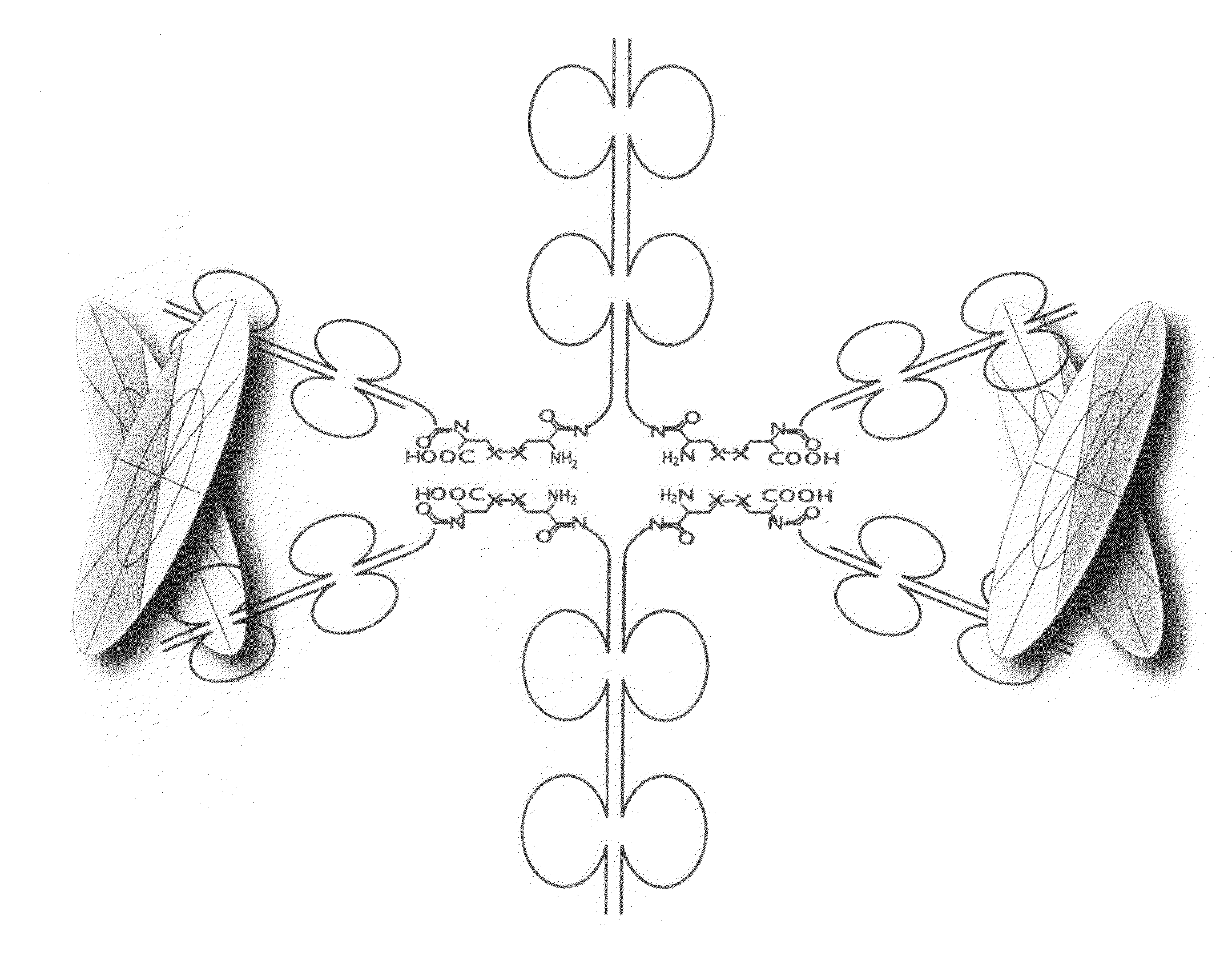
Hybrid immunoglobulins with moving parts
a technology of immunoglobulins and moving parts, which is applied in the field of hybrid immunoglobulins with moving parts, can solve the problems of inflexible connections between the two binding domains that do not provide machine-like motion, and the number of parts or connections in any machine is counterproductive, and numerous attempts to engineer antibodies and immunoadhesins that bind symmetrically have failed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Immunoglobulin Fc with N-Terminal-S-Termini (S-Fc)
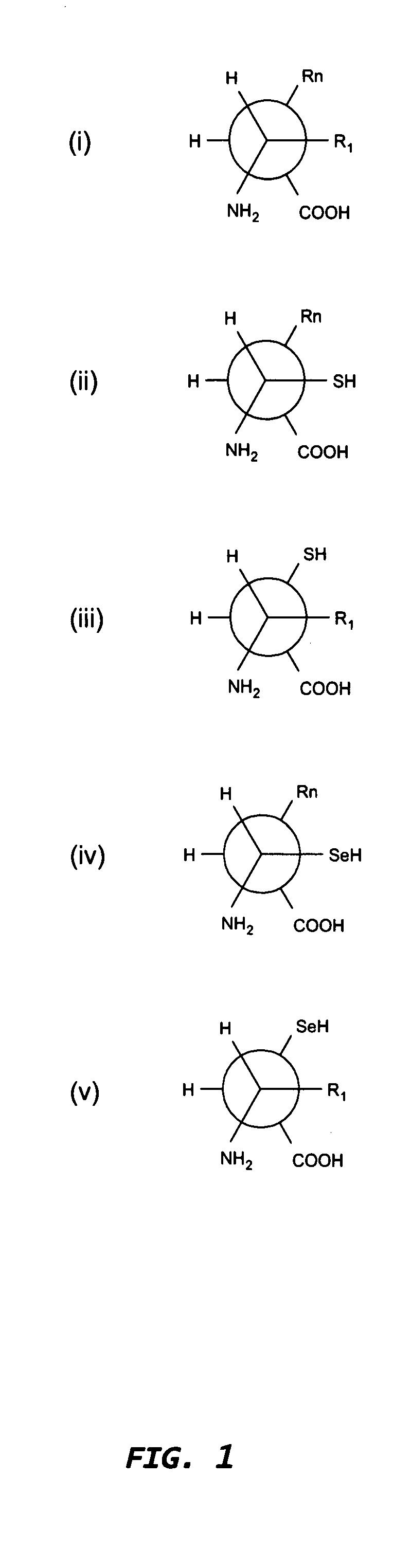
[0380]Digestion of immunoglobulin (IgG) with papain yields two Fab fragments and one Fc fragment (Porter (1959) Biochem. 73, 119-126). The site of proteolysis in human IgG is the heavy chain hinge region between the cys-5 and cys-11 residues, EPKSCDKTHTCPPCP, (Fleischman et al., Biochem J. (1963) 88, 220-227; Edelman et al. (1969) Proc. Natl. Acad. Sci. 63, 78-85). The cys-5 residue normally forms a disulfide bond with the human IgG light chain, which is easily cleaved under mild reducing conditions, making it an ideal candidate for Fc-like molecules with N-terminal-S-termini (S-Fc).
[0381]Accordingly, host cells were transfected with expression vectors encoding IgG1 pre-Fc chimeric polypeptides consisting of a signal peptide joined at its C-terminus by a peptide bond to the N-terminus of an Fc domain beginning at the cys-5 residue, CDKTHTCPPCP (FIG. 35A). The heterologous signal peptides used are selected from proteins...
example 2
Preparation of Immunoglobulin Fc with N-Terminal-X-Termini (X-Fc)
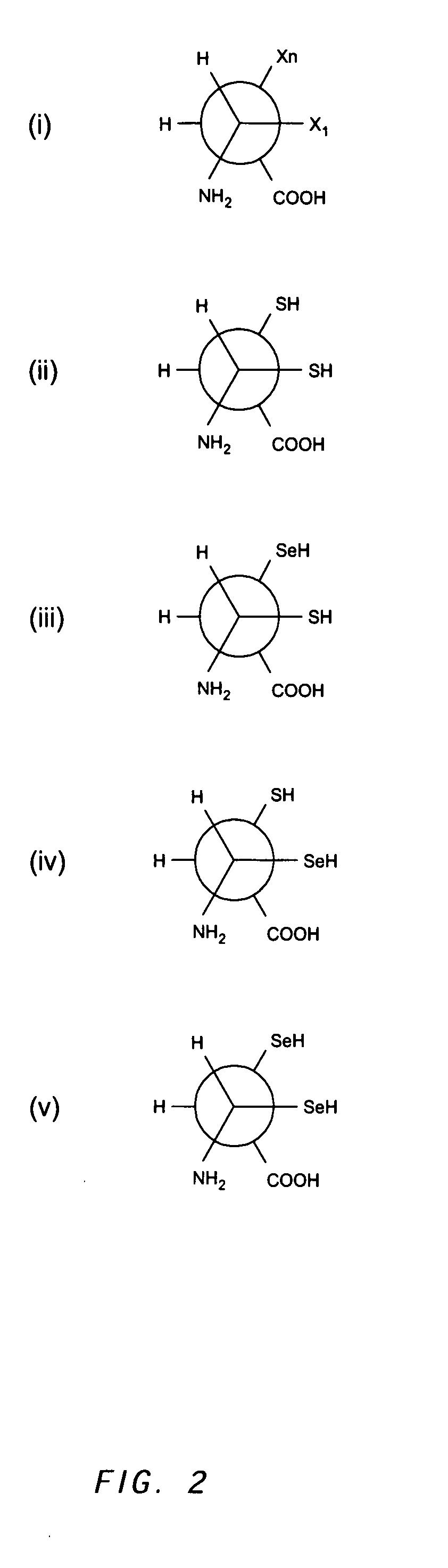
[0410]Selenocysteine (sec) is the 21st amino acid incorporated during ribosome-mediated protein synthesis (Zinoni et al. (1986) Proc. Natl. Acad. Sci. 83, 4650-4654; Chambers et al. (1986) EMBO J. 5, 1221-1227). The process is complex and distinct from cysteine incorporation, requiring an mRNA selenocysteine insertion element in order to decode a UGA stop codon. Protein semi-synthesis offers an alternative means for preparing Fc-like molecules having N-terminal-X-termini (X-Fc) that begin at cysteine (cys) and / or selenocysteine (sec).
[0411]Accordingly, host cells are transfected with constructs that encode pre-Fc chimeric polypeptides consisting of a signal peptide joined at its C-terminus by a peptide bond to the N-terminus of an Fc domain beginning at cys-11, CDKTHTCPPCP (FIG. 36A) and cys-14 CDKTHTCPPCP (FIG. 36B). The heterologous signal peptides used are selected from proteins with N-terminal cysteines (part i). T...
example 3
Preparation of Immuoglobulin Fc with C-Terminal-X-Termini (Fc-X)
[0421]IgG is expressed in two abundant forms, the soluble antibody molecule and the cell-bound B-cell receptor. Both forms arise from a single messenger RNA by alternative splicing with the result that two additional exons are added to the IgG heavy chain coding region (Tyler et al. (1982) Proc. Natl. Acad. Sci. 79, 2008-2012; Yamawaki-Kataoka et al. (1982) Proc. Natl. Acad. Sci. 79, 2623-2627). The first exon added (M1 exon) encodes a stretch of 18 amino acids, ELQLEESCAEAQDGELDG, which flexibly tethers IgG to the cell surface, making it a good choice for novel Fc-like molecules with a C-terminal-X-terminus (Fc-X). The C-terminal gly-18 residue of the M1 domain is also well-suited for preparing Fc-intein fusion proteins used in generating a C-terminal activated thioester. Following an intein autocleavage reaction, a thioester intermediate is generated that permits the facile addition of cysteine or selenocysteine to th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bond | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com