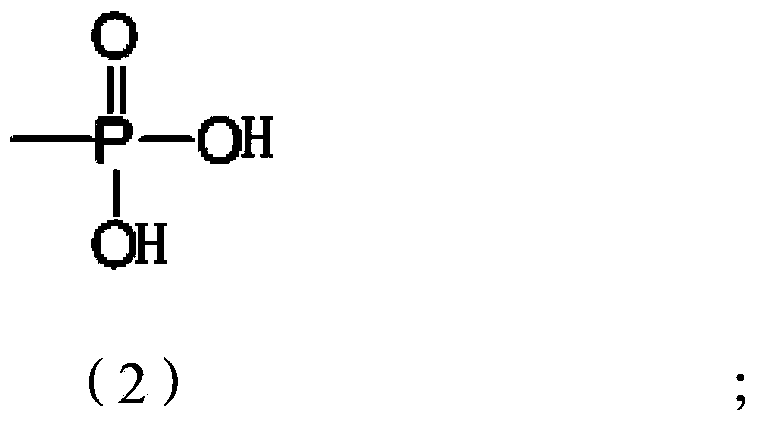Tanshinone IIA phosphate phenolic ester derivative and preparation process thereof
A technology of phenolic phosphate and tanshinone, which can be used in drug combinations, pharmaceutical formulations, steroids, etc., can solve the problems of difficult biofilm structure system, unsuitable for the treatment of cerebrovascular diseases, etc., and achieve good water solubility and broad application value. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
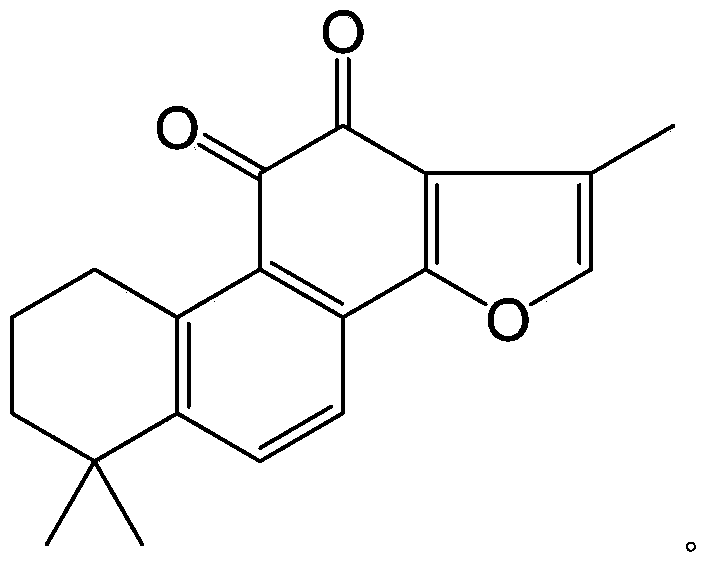
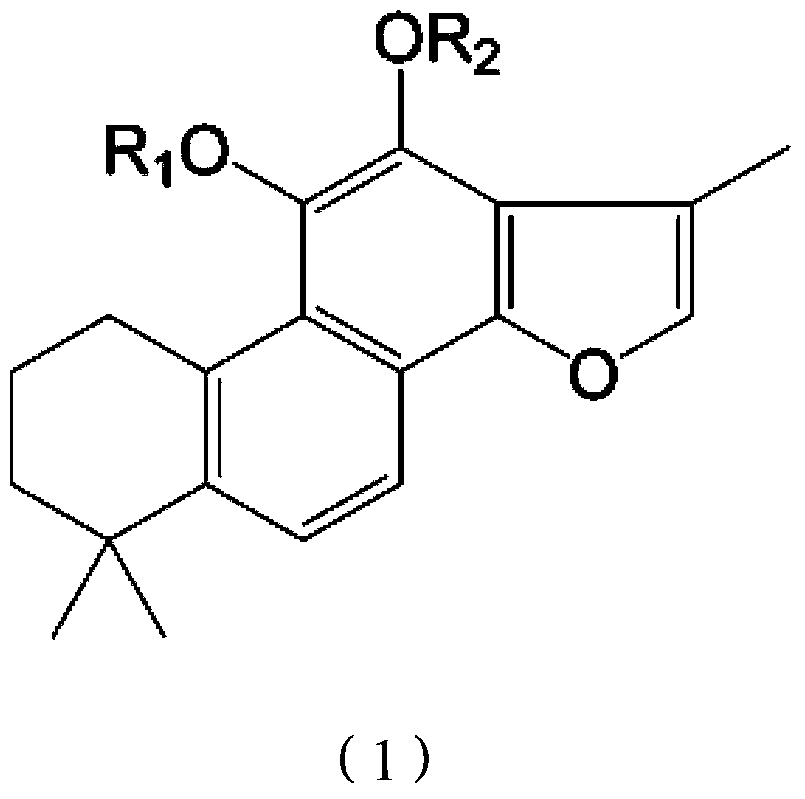
[0056] Weigh 2.94g of tanshinone IIA raw material into a 100ml three-necked bottle, add about 160mg of 10% palladium carbon (Pd / C), dissolve in about 30ml of anhydrous THF, stir at room temperature under the protection of hydrogen until the color of the solution fades. First add about 5.8ml of triethylamine into the reaction flask, and slowly add 8.7ml of diethylphosphoryl chloride dropwise under cooling in an ice-water bath. After the dropwise addition, the mixture was stirred at room temperature for 24 hours to terminate the reaction. The reaction solution was diluted with ethyl acetate and poured into 100 ml of ice water. After standing still, the organic layer was separated, and the aqueous layer was extracted 1-2 times with an equal volume of ethyl acetate, and the organic layers were combined, washed with water, and anhydrous Na 2 SO 4 Dry and evaporate to dryness to obtain the intermediate of tanshinone ⅡA phosphoryl diethyl ester.
[0057] The compound was dissolved ...
Embodiment 2
[0064] Weigh 2.94g of tanshinone IIA raw material into a 100ml three-neck flask, add about 300mg of 5% Pd / C, dissolve in about 50ml of anhydrous ethyl acetate, stir at room temperature under the protection of hydrogen until the color of the solution fades. 500 mg of sodium hydride was added to the reaction flask, and after reacting at room temperature for 2 hours, 9.5 g of dibenzylphosphoryl chloride was slowly added dropwise under cooling in an ice-water bath. After the dropwise addition, stir at room temperature for 12 hours, pour the reaction solution into 100 ml of ice water, let stand and separate the organic layer, and extract the water layer with an equal volume of ethyl acetate for 1-2 times. Combined organic layers, washed with water, anhydrous Na 2 SO 4 Dry and evaporate to dryness to obtain the intermediate of tanshinone ⅡA phosphoryl dibenzyl ester. This compound was dissolved in 50ml of anhydrous methanol, about 500mg of 5% Pd / C was added, and hydrogen gas was p...
Embodiment 3
[0071] Weigh 2.94g of tanshinone IIA raw material into a 100ml three-neck flask, add about 160mg of 10% Pd / C, dissolve in about 30ml of anhydrous THF, stir at room temperature under the protection of hydrogen until the color of the solution fades. First add about 3ml of triethylamine into the reaction flask, then slowly add about 5.5g of dibenzylphosphoryl bromide dropwise under cooling in an ice-water bath, and control the reaction temperature at about 25°C. After the dropwise addition was completed, the mixture was stirred at room temperature for 10 hours. The reaction solution was diluted with 30 ml of ethyl acetate and poured into 100 ml of ice water. After standing still, the organic layer was separated, and the aqueous layer was extracted 1-2 times with an equal volume of ethyl acetate, and the organic layers were combined, washed with water, and anhydrous Na 2 SO 4 Dry and evaporate to dryness to obtain the intermediate of tanshinone ⅡA monophosphoryl dibenzyl ester. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com