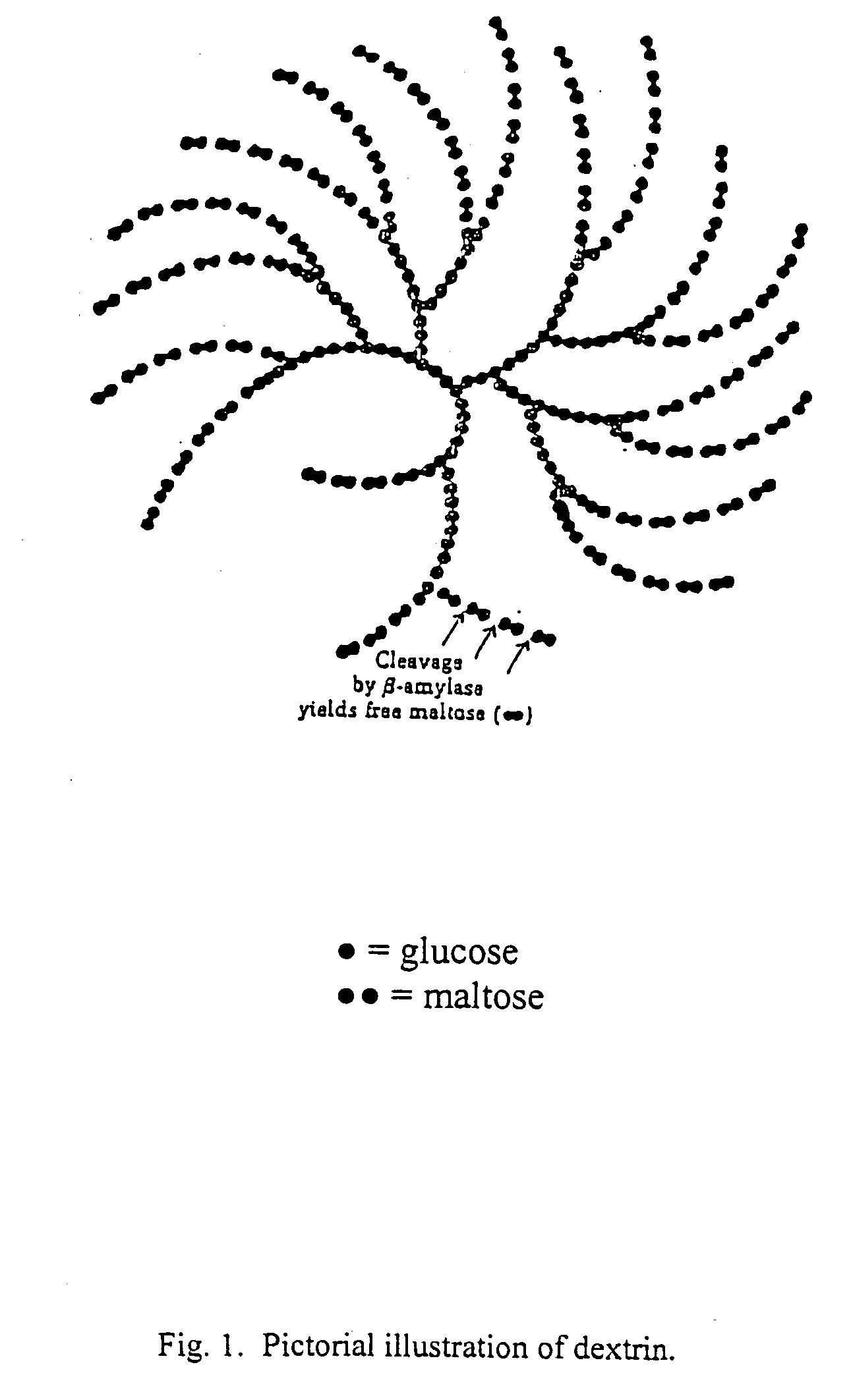
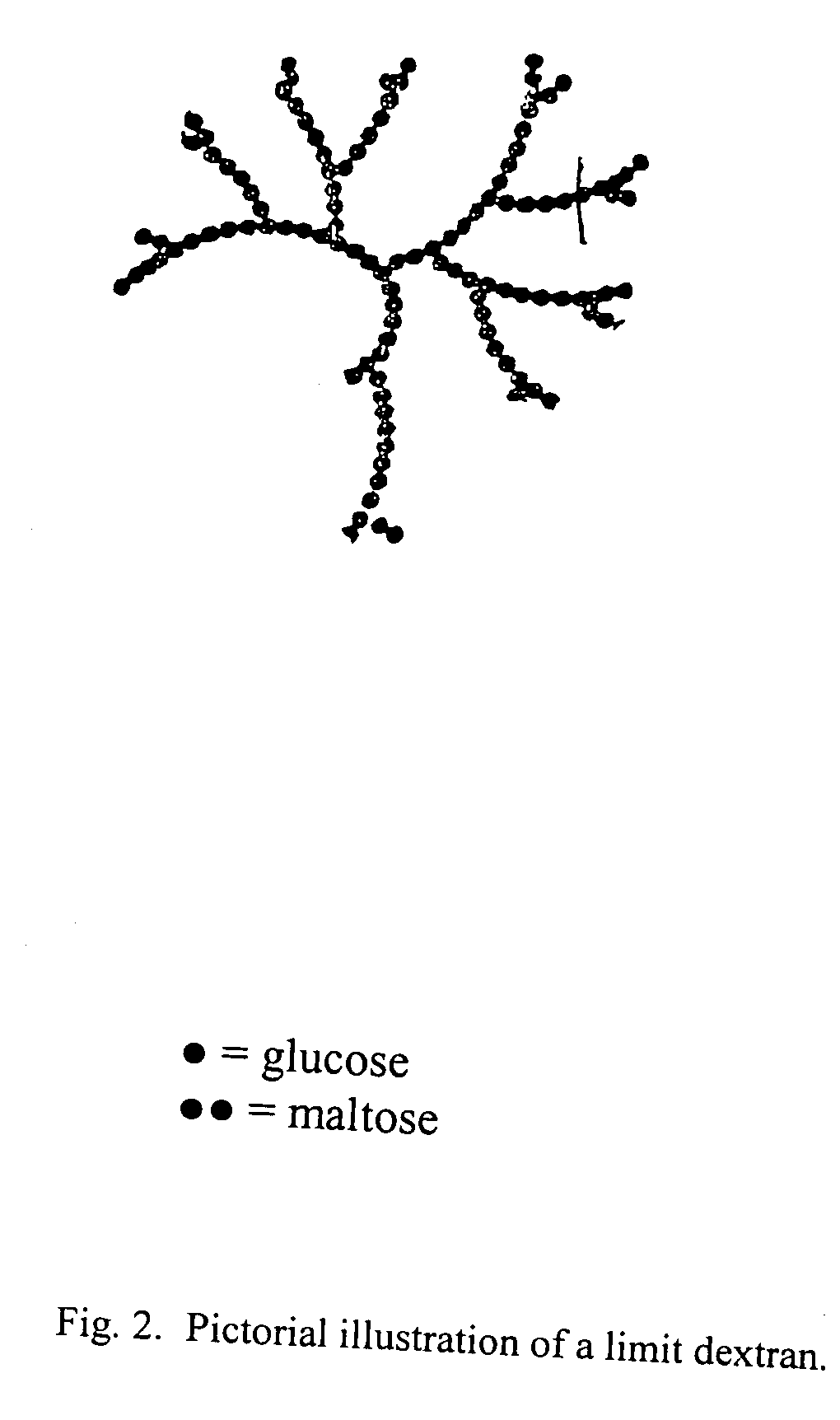
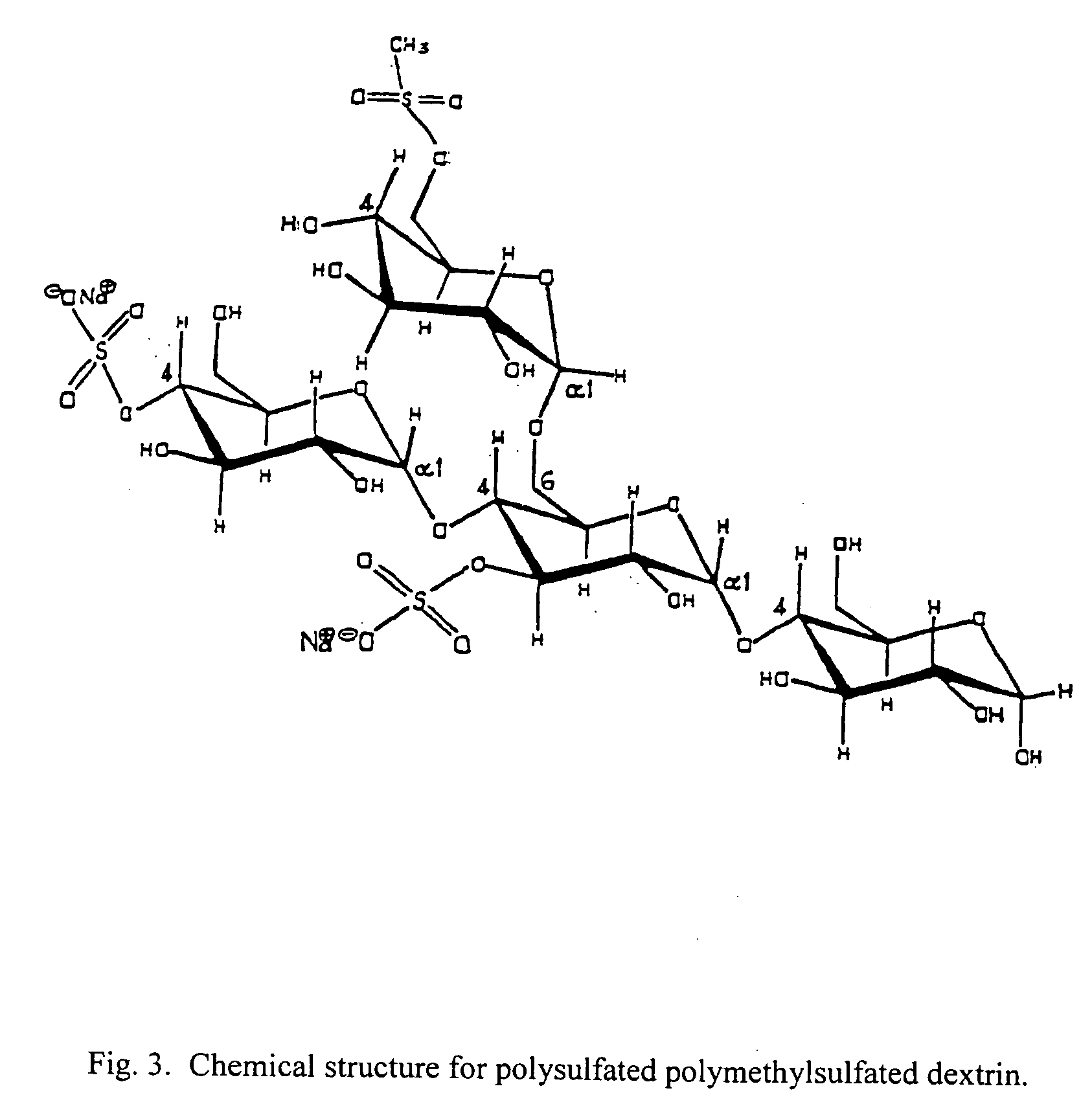
Antiviral composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Purification of Dextrin
[0064] Type I corn starch dextrin of USP grade having a molecular weight distribution of approximately 30% of 2,000 to 4,000 daltons and 60% of 8,000 to 10,000 daltons as determined by gel permeation chromatography is supplied. The dextrin is purified by dissolving into sufficient purified water and dialyzing against purified water. The dialysis membrane has a pore size of 3000 to 6000 daltons so that smaller size dextrin and impurities are eliminated. The purified starting material is then dried by lyophilization and is obtained as a white fluffy solid, melting point 266-274° C. with decomposition.
example 2
Synthesis of Polysulfate Polymethylsulfate Dextrin
[0065] To 10 mL of dry pyridine is added 1.0 mL of methanesulfonyl chloride and 1.0 mL of chlorosulfonic acid. To this is added 500 mg of dextrin. The mixture is heated to 55° C. for a period of twelve hours. Ten grams of sodium hydroxide in 100 mL of water is then added. The aqueous layer is transferred to a dialysis membrane and dialysed against water until the pH is neutral. The polysulfate polymethylsulfate dextrin is obtained as a fluffy white solid by removal of the water by lyophylization. Weight 455 mg; melting point 185-215 degrees Celsius with decomposition 215-220 degrees Celsius. Elemental analysis shows carbon 31.27%, hydrogen 6.38%, and sulfur 11.28%. The 300 MHZ NMR in deuterium shows a broad singlet at 5.8 to 5.4 ppm and a broad quartet at 4.5 to 3.2 ppm.
[0066] The methysulfate group may be added to any other sulfates that have been tested for antiviral activity such as dextrin sulfate, dextran sulfate, cyclo-dextri...
example 3
Synthesis and Purification of Polysulfate Polymethylsulfate Dextrin from Sulfated Dextrin
[0067] To 10 mL of clean dry pyridine is added 1.0 mL of methanesulfonyl chloride. The addition requires stirring and cooling. This mixture is then heated to 55° C. To this is added 500 mg of sulfated dextrin with stirring. The mixture is heated to 55° C. and stirred for a period of twelve hours. The mixture is then cooled and ten grams of cooled sodium hydroxide in 100 mL of water is slowly added with stirring and cooling.
[0068] The aqueous layer is allowed to separate and is transferred to a dialysis membrane and dialyzed against purified water until the pH of the water remains neutral. The polysulfate polymethylsulfate dextrin is obtained as a solid by removal of the water by lyophilization.
[0069] The above synthesis can be applied to any form of sulfated dextrin such as dextrin-2-sulfate, dextrin-3-sulfate, dextrin-6-sulfate or multiple sulfates. Any molecular weight of sulfate dextrin ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Transmission | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com