Building method of ulcerative colitis transformation animal model
A technology of ulcerative colitis and animal models, applied in the direction of pharmaceutical formulations, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of increased IL-17 levels, and achieve the effect of increasing expression and increasing the number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
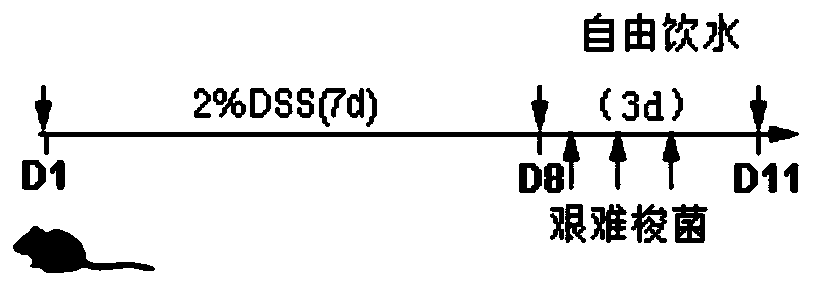
[0037] The construction method of the ulcerative colitis transformation animal model of the present embodiment comprises the following steps:
[0038] 1. Experimental materials
[0039] 1. Experimental animals: 40 male C57BL / 6J mice, SPF grade, age 5wk, body weight 18-20g, purchased from Shanghai Slack Animal Center.
[0040] 2. Strains: C.difficile ATCC 43255 purchased from American type culture collection (ATCC)
[0041] 3. Main experimental reagents
[0042] Dextran sodium sulfate (DSS) (purchased from MP Biomedicals), FITC-CD4 antibody, PerCP-Cy5.5-CD3e antibody, PE-IFN-γ antibody, Fixation Buffer (both purchased from Biolegend); APC -IL-17A antibody (both purchased from ebosicence company); PMA (purchased from Sigmaaldrich company), Ionomycin ionomycin (purchased from aladdin company), 5% FBS 1640 medium (purchased from Gibco company), mouse IL-17A ELISA Kit (purchased from Shanghai Jitai Yikesai Company), erythrocyte lysate (purchased from Beyontian Company), PBS buff...
Embodiment 2
[0064] Example 2: Observation indicators
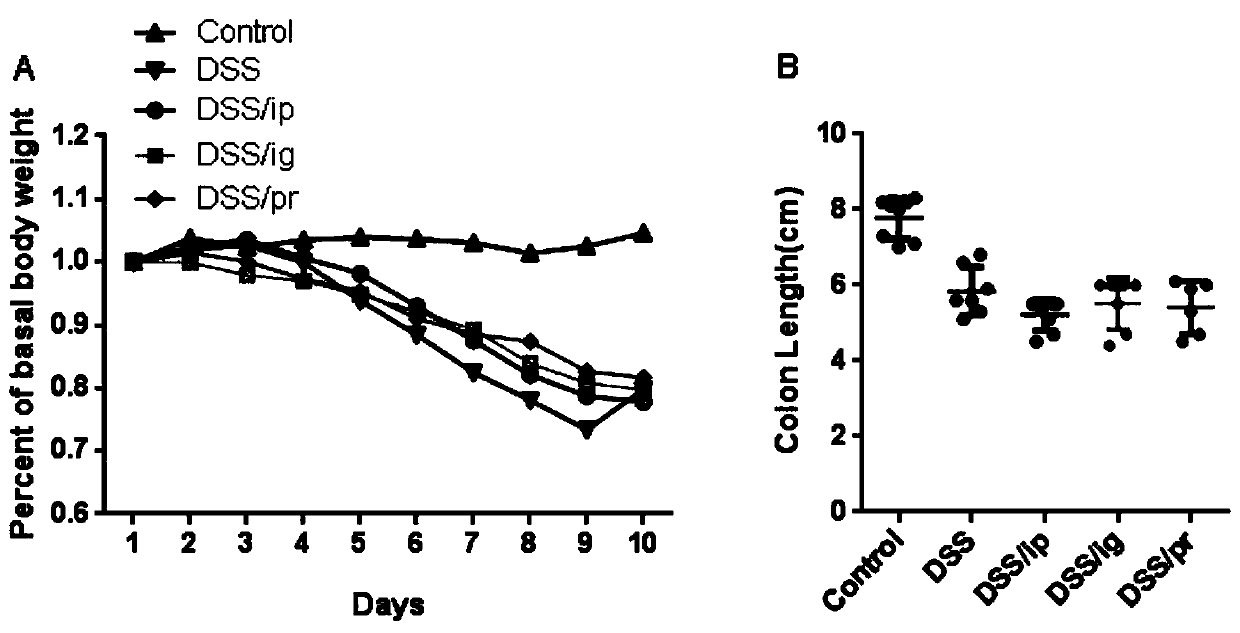
[0065] Observe and record the daily body weight changes of the mice given 2% DSS from the 1st day to the 10th day, and record the colon length on the 11th day.
Embodiment 3
[0066] Embodiment 3: Determination of biochemical indicators
[0067] 1.1 Determination of Th1 and Th17 ratios of lymphocytes in spleen and mesenteric lymph nodes by flow cytometry
[0068] 1) Take out the mesenteric lymph nodes and spleen tissues placed in PBS with 2% FBS, transfer them to a 40um blue sieve, place a sterile six-hole plate under the sieve, grind the mesenteric lymph nodes and spleen respectively with a 2.5ml syringe rubber stopper, And wash the screen with 4ml of PBS containing 2% FBS, transfer the filtrate to a 10ml sterile centrifuge tube, and centrifuge at 800g*20min*4°C.
[0069] 2) After the centrifugation, discard the supernatant, and resuspend the spleen in 1ml erythrocyte lysate, lyse on ice for 10 minutes and centrifuge at 800g*5min*4°C; directly resuspend the lymph nodes in 500ul PBS containing 2% FBS, temporarily at 4°C live.
[0070] 3) After the centrifugation of the spleen, discard the supernatant, resuspend in 500ul PBS containing 2% FBS, temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com