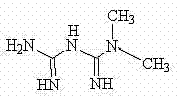
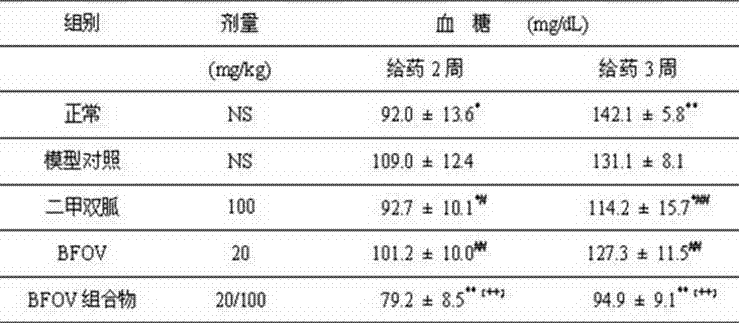
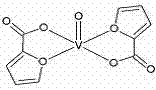
Bis(alpha-furancarboxylato)oxovanadium composition for treatment of diabetes
A technology of furocarboxylic acid and diabetes drug, which is applied in the directions of drug combination, medical preparations containing active ingredients, metabolic diseases, etc., can solve the problems such as insufficient control of blood sugar, complex etiology and pathogenesis, and induction of cardiovascular diseases, etc. To achieve the effect of improving drug stability, accurate dosage and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 - tablet:
[0061] 1.1 [Prescription] BFOV 10 g, metformin 50 g, lactose 30 g, microcrystalline cellulose 90 g, micronized silica gel 3 g, magnesium stearate 1 g, low-substituted hydroxypropyl cellulose 10 g, povidone 30 6 g.
[0062] [Preparation] Put BFOV, metformin, lactose, povidone, microcrystalline cellulose, etc. in a dry granulator and stir, and the obtained granules are mixed with magnesium stearate and low-substituted hydroxypropyl cellulose and passed through a 16-mesh sieve. The granules prepared by the above method are compressed into disc-shaped or heterogeneous tablet-shaped preparations, including sugar-coated tablets, film tablets and the like. Tablet weight: 0.1g / tablet.
[0063] 1.2 [Prescription] BFOV 10 g, metformin 200 g, lactose 40 g, microcrystalline cellulose 110 g, micropowder silica gel 6 g, magnesium stearate 2 g, low-substituted hydroxypropyl cellulose 20 g, povidone 30 12 g.
[0064] [Preparation] Put BFOV, metformin, lactose...
Embodiment 2
[0067] Embodiment 2——granule:
[0068]2.1 [Prescription] Bis(α-furanoic acid) vanadyl (BFOV) 10 g, metformin 50 g, sodium saccharin 10 g, croscarmellose sodium 10 g, microcrystalline cellulose 75 g, micronized silica gel 3 g, Sucrose fine powder 15 g, orange flavor 6 g, sodium bicarbonate 15 g, sodium lauryl sulfate 6 g.
[0069] [Preparation] After passing BFOV, metformin and sucrose fine powder through a 16-mesh sieve, put it in a mixer and mix it with sodium saccharin. The mixture is granulated with povidone ethanol solution, and after drying, it is sieved through 30 meshes to sieve the whole granule and mix with the remainder of the prescription. Sodium bicarbonate is passed through a 30-mesh sieve, orange flavor and sodium lauryl sulfate are passed through a 60-mesh sieve. Whole grain, graded. Loading capacity 0.1g / bag.
[0070] 2.2 [Prescription] BFOV 10 g, metformin 200 g, saccharin sodium 10 g, croscarmellose sodium 20 g, microcrystalline cellulose 94 g, micropowde...
Embodiment 3
[0074] Embodiment 3——capsules:
[0075] 3.1 [Prescription] BFOV 10 g, metformin 50 g, micronized silica gel 3 g, magnesium stearate 1 g, microcrystalline cellulose 136 g.
[0076] [Preparation] Pass the fine powder of BFOV, metformin and dressing through a 16-mesh sieve, and fill it into hollow capsules. According to the nature of the packaging material, it can be divided into hard capsules or enteric-coated capsules. Capsule weight: 0.1 g / capsule.
[0077] 3.2 [Prescription] BFOV 10 g, metformin 200 g, micronized silica gel 6 g, magnesium stearate 2 g, microcrystalline cellulose 182 g.
[0078] [Preparation] Pass the fine powder of BFOV, metformin and dressing through a 16-mesh sieve, and fill it into hollow capsules. According to the nature of the packaging material, it can be divided into hard capsules or enteric-coated capsules. Capsule weight: 0.2 g / capsule.
[0079] 3.3 [Prescription] BFOV 10 g, metformin 500 g, micronized silica gel 15 g, magnesium stearate 5 g, mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com