Lixisenatide controlled-release microspheres and preparation method thereof
A technology of sustained-release microspheres and lixisenatide, applied in the field of medicine, can solve the problems of unfavorable disease control, peak and valley of drug concentration, poor compliance with treatment, etc., and achieve stable and sustained drug release, good tissue compatibility, In vitro release smooth effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Preparation of lixisenatide sustained-release microspheres of the present invention
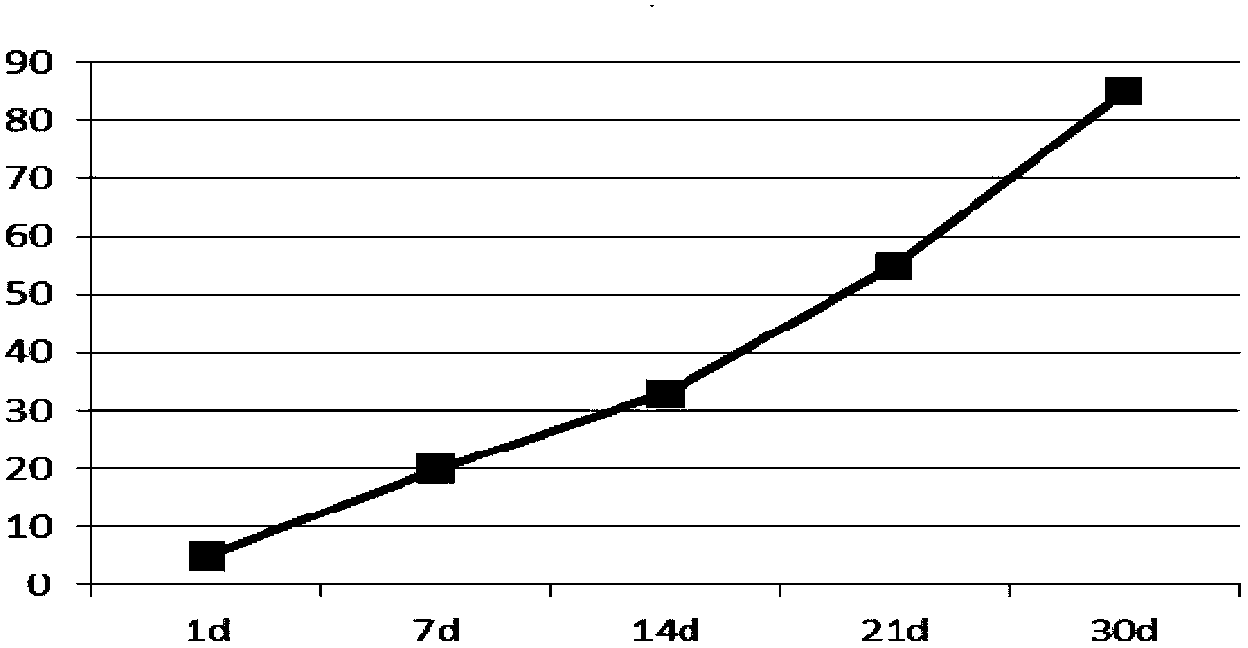
[0044] Dissolve 800mg of PLGA (LA:GA=50:50, Mw=10000) in 3.0ml of dichloromethane to make the oil phase, and dissolve 50mg of lixisenatide in 0.5ml of water for injection (containing 12.5mg of gelatin) to form the inner phase Water phase, add it to the above oil phase, ultrasonic emulsification to form W / O colostrum, put 50ml of pVA solution containing 2% (mass percentage) in a stirring container, and quickly add colostrum under high-speed stirring (5000rpm) Fully homogenize in the external water phase to obtain double emulsion. After three minutes, slow down the rotation speed to 300rpm to stir the double emulsion, stir at room temperature for 4 hours, separate and wash the microspheres after hardening, and freeze-dry them. Lixisenatide microspheres The encapsulation rate is 88%, 45μm≤particle size≤65μm.
[0045] Determination of the release rate of lixisenatide sustain...
Embodiment 2
[0046] Embodiment 2: Preparation of lixisenatide sustained-release microspheres of the present invention
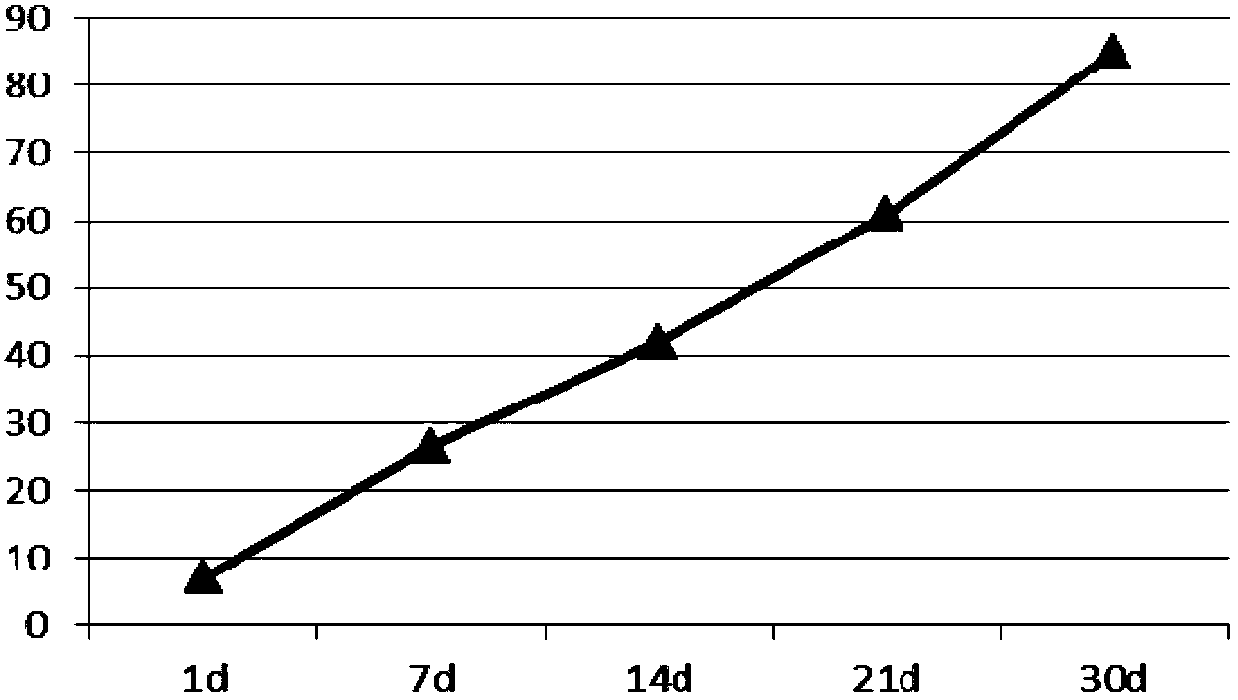
[0047] Dissolve 250mg of PLGA (LA:GA=25:75, Mw=5000) in 5ml of ethyl acetate to form an oil phase, dissolve 29mg of lixisenatide in 0.75ml of water for injection (containing 12mg of gelatin) to form an inner water phase, Add it to the above oil phase, ultrasonic emulsification, to form W / O colostrum, put 50ml of pVA solution containing 3% (mass percentage) and 0.01% poloxamer solution in a stirring container, and put the colostrum under high-speed stirring (7000rpm) quickly added to the external water phase and fully homogenized to obtain double emulsion. After three minutes, slow down the speed (400rpm) to stir the double emulsion. Stir at 10-12°C for 4 hours. After hardening, the microspheres were separated and washed, and freeze-dried That is, the encapsulation efficiency of lixisenatide microspheres is 89%, and 45 μm≤particle size≤65 μm.
[0048] Determination of the...
Embodiment 3
[0049] Embodiment 3: Preparation of lixisenatide sustained-release microspheres of the present invention
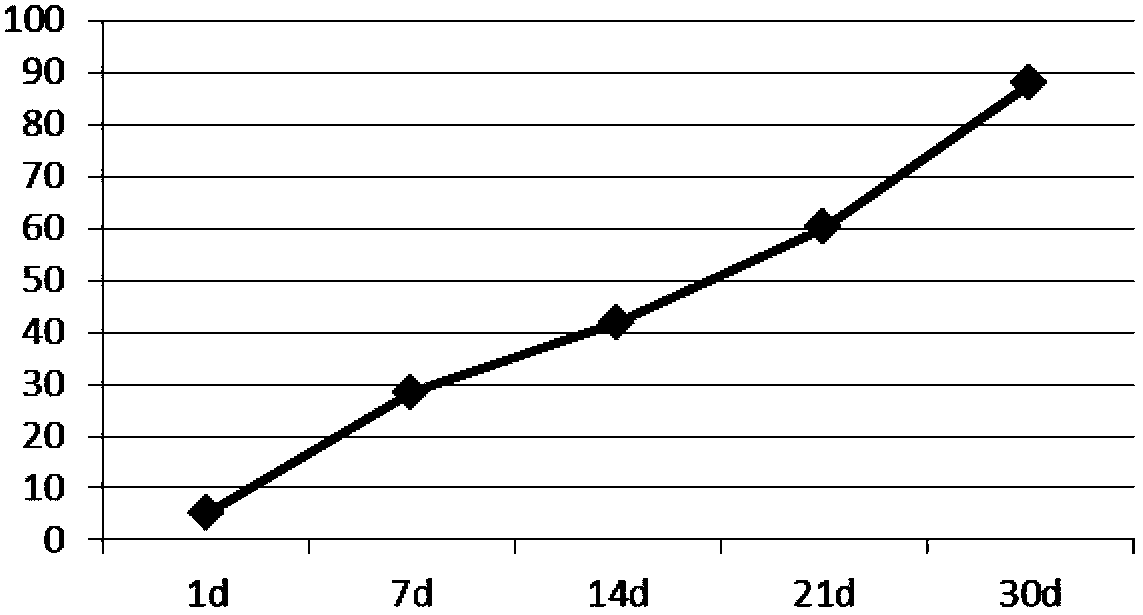
[0050] Dissolve 96mg of PLGA (LA:GA=20:80, Mw=20000) in 1.0ml of dichloromethane to make the oil phase, dissolve 12mg of lixisenatide in 1.5ml of water for injection (containing 12.0mg of gelatin) to form the inner Water phase, add it to the above oil phase, ultrasonic emulsification to form W / O colostrum, put 50ml of 1% (mass percentage) pVA and 0.4% sodium chloride solution in a stirring container, and stir the colostrum at high speed Quickly add (3000rpm) into the external water phase to fully homogenize the double emulsion. After three minutes, slow down the rotation speed (50rpm) to stir the double emulsion, stir at 8-15°C for 4 hours, separate and wash the microspheres after hardening, and freeze Just dry, the encapsulation rate of lixisenatide microspheres is 90%, 45μm≤particle size≤65μm.
[0051] Determination of the release rate of lixisenatide sustained-release...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com