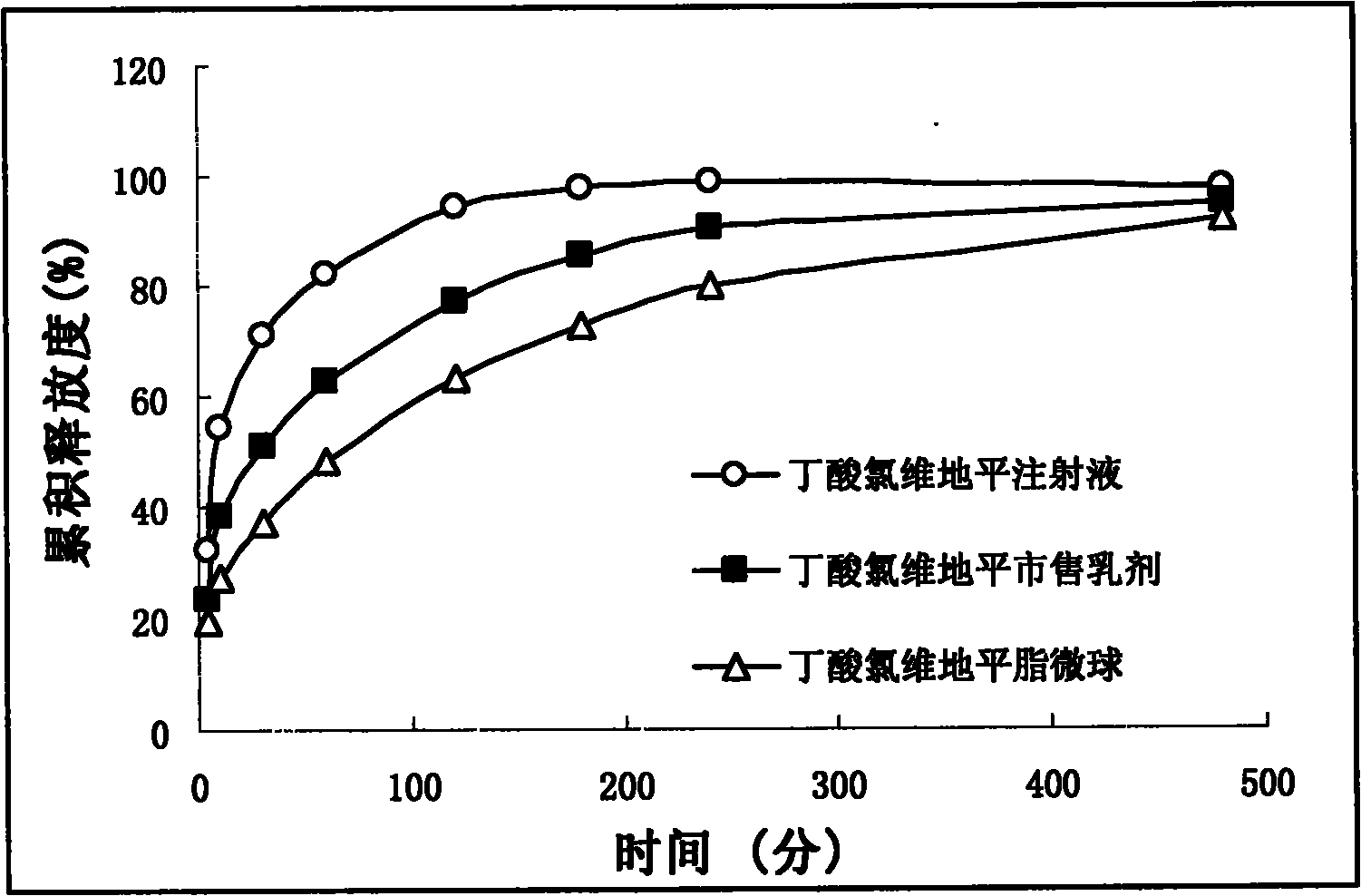
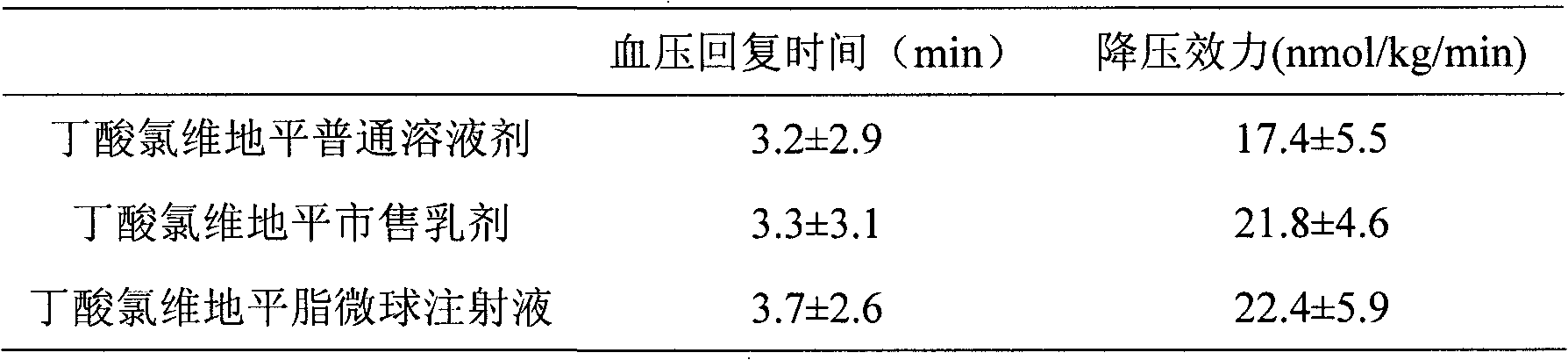
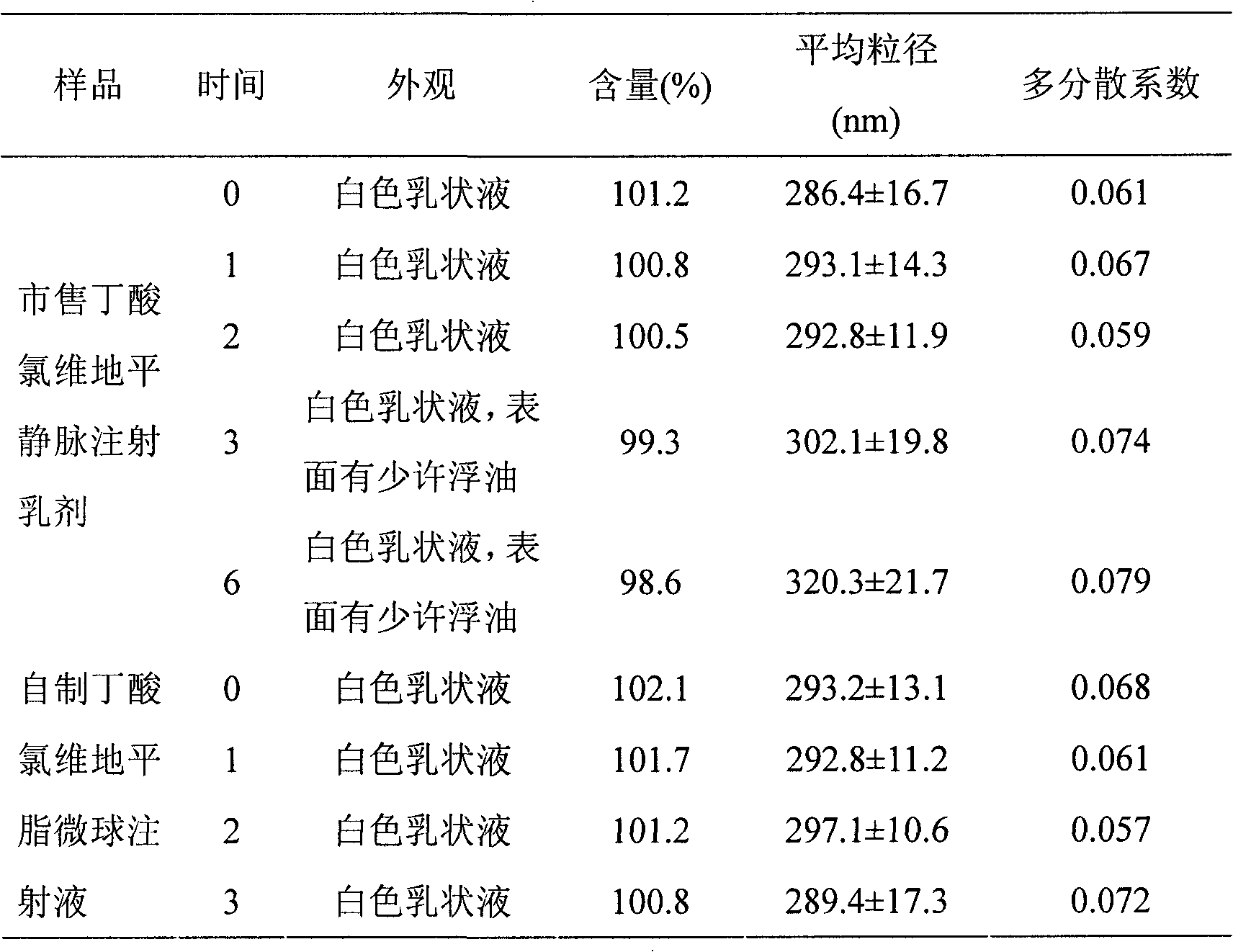
Butyrate clevidipine lipid microsphere injection and preparation method thereof
A technology of clevidipine butyrate and clevidine butyrate, applied in the directions of liquid delivery, pharmaceutical formulation, emulsion delivery, etc., to achieve the effects of improving stability, improving solubility, and avoiding cracking and bleeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] prescription:
[0053]
[0054] w / w(%)
[0055]
[0056] Clevidipine butyrate 0.01
[0057] soybean oil 10
[0058] Soy Lecithin 1.5
[0059] Macrogol 1000 Vitamin E Succinate 0.4
[0060] Glycerin 2.3
[0061] Sodium hydroxide to adjust the pH to 5.0-8.0
[0062] water for injection balance
[0063]
[0064] Preparation:
[0065] ① Add clevidipine butyrate and soybean lecithin into soybean oil, stir and dissolve at 35-80°C, and mix evenly to obtain an oil phase.
[0066] ②Add polyethylene glycol 1000 vitamin E succinate and glycerin into water for injection, stir and dissolve at 20-80°C to obtain an aqueous phase.
[0067] ③ At 30-75°C, mix and shear the water phase and oil phase to form colostrum.
[0068] ④ Pass the colostrum through a high-pressure homogenizer, ...
Embodiment 2
[0074] prescription:
[0075]
[0076] w / w(%)
[0077]
[0078] Clevidipine butyrate 0.03
[0079] fish oil 20
[0081] Glycerin 2.0
[0082] Macrogol 2000 Stearate 0.6
[0083] Vitamin E 0.5
[0084] Disodium hydrogen phosphate to adjust the pH to 5.0-8.0
[0085] water for injection balance
[0086]
[0087] Preparation:
[0088] ①Add clevidipine butyrate, egg yolk lecithin and vitamin E into the fish oil, stir and dissolve at 35-80°C, mix well to obtain the oil phase.
[0089] ②Add polyethylene glycol 2000 stearate and glycerin into water for injection, stir and dissolve at 20-80°C to obtain an aqueous phase.
[0090] ③ At 30-75°C, mix and shear the water phase and oil phase to form colostrum.
[0091] ④ Pass the colostrum through a high-pressure homogenizer, adjust ...
Embodiment 3
[0097] prescription:
[0098]
[0099] w / w(%)
[0100]
[0101] Clevidipine butyrate 0.05
[0102] olive oil 10
[0103] Hydrogenated Lecithin 3.0
[0104] Solutol HS 15 2.0
[0105] Glycerin 2.5
[0106] water for injection balance
[0107]
[0108] Preparation:
[0109] ①Add clevidipine butyrate and hydrogenated lecithin into olive oil, stir and dissolve at 35-80°C, and mix evenly to obtain an oil phase.
[0110] ②Add Solutol HS 15 and glycerin to water for injection, stir and dissolve at 20-80°C to obtain an aqueous phase.
[0111] ③ At 30-75°C, mix and shear the water phase and oil phase to form colostrum.
[0112] ④ Pass the colostrum through a high-pressure homogenizer, adjust the pressure to 500-1000 bar, and homogenize for 5-15 times under high pressure to obtain lipid microsphere injection.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com