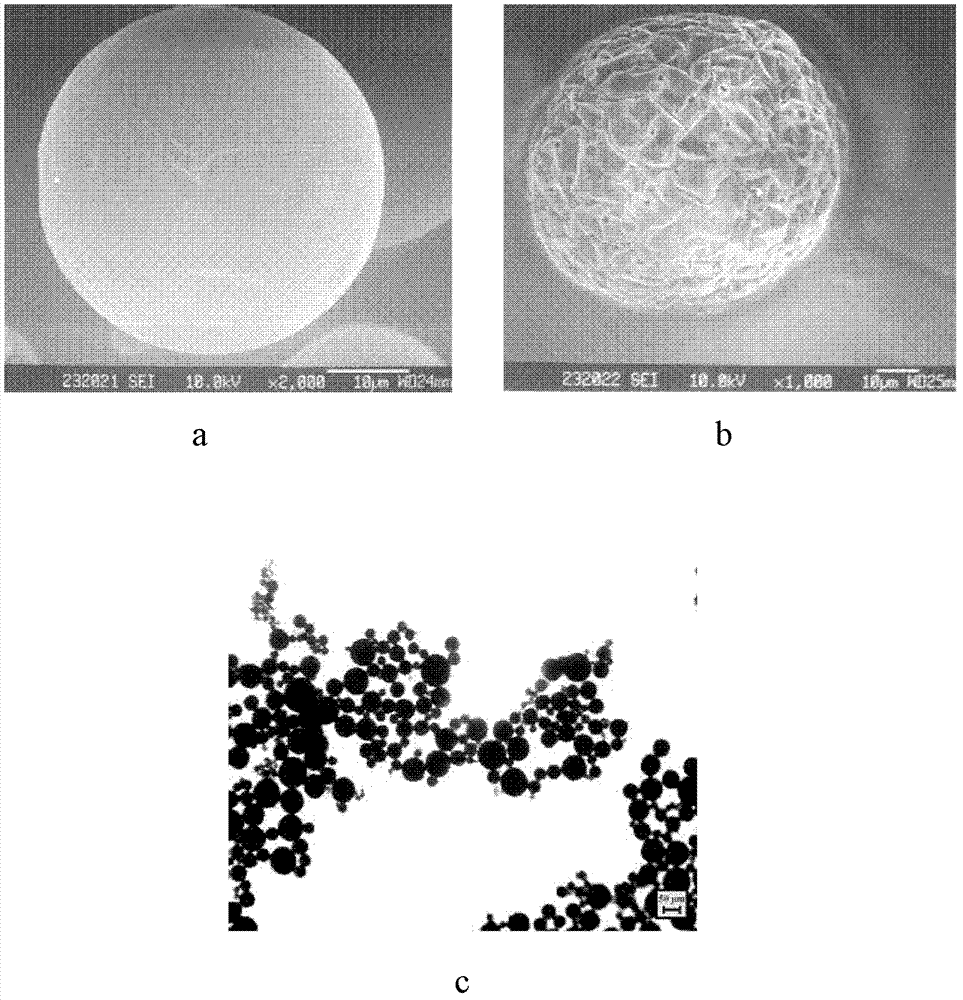
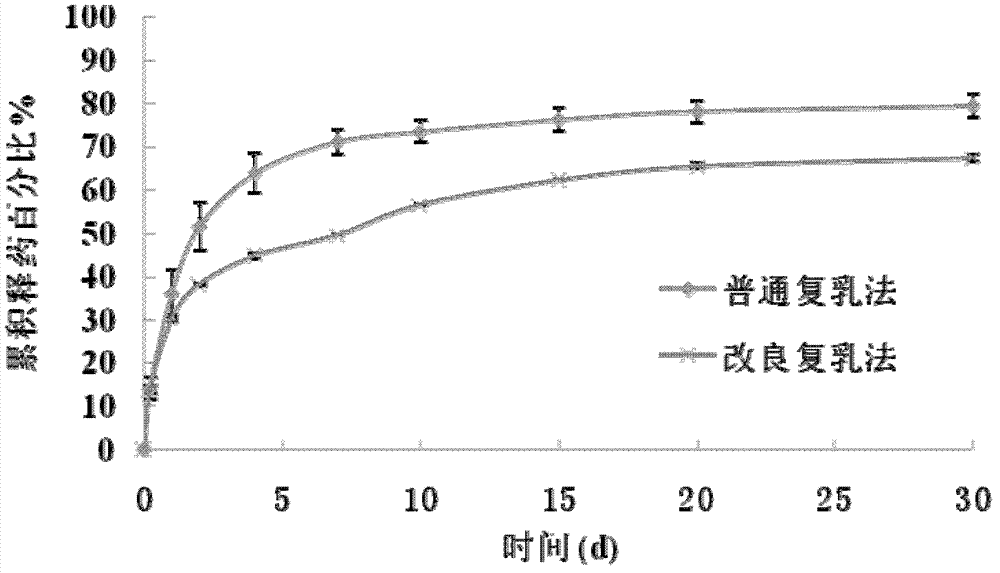
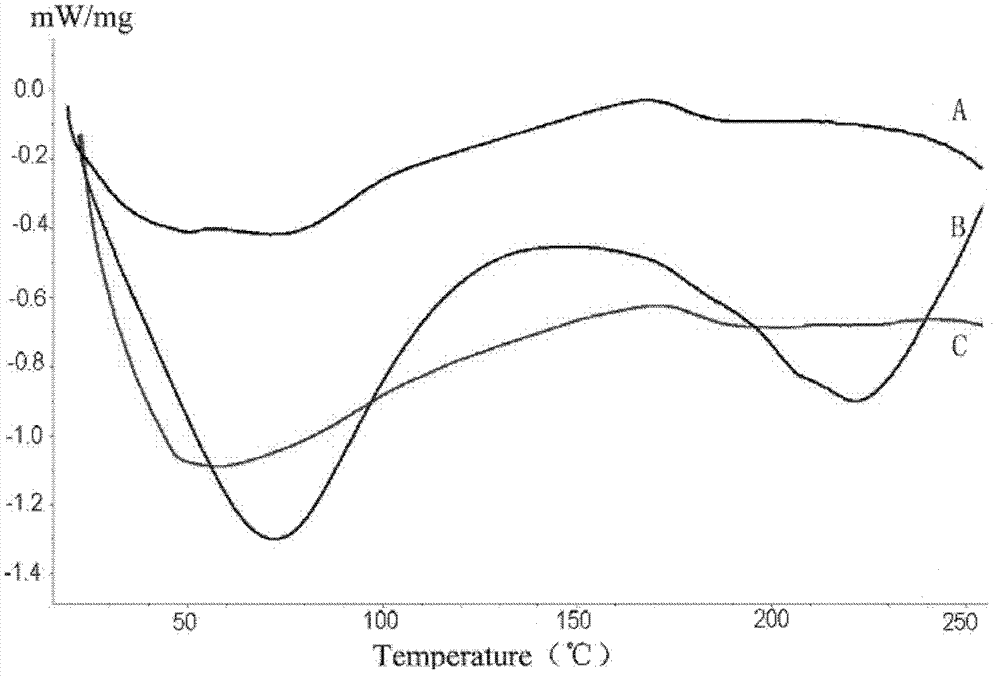
Protein-medicament-carrying PLGA composite microspheres and preparation method thereof
A technology of composite microspheres and proteins, which is applied in the fields of peptide/protein components, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve the problems of difficult to meet treatment needs, obvious drug burst release phenomenon, and low blood drug concentration levels. , to prolong the action time of the drug, the preparation process is stable, and the fluidity is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 interferon PLGA composite microsphere
[0046] (1) Preparation of PLGA solution
[0047] 600 mg PLGA was dissolved in 4 ml dichloromethane to form an organic phase (15% g / ml).
[0048] (2) Preparation of interferon sodium alginate solution
[0049] Dissolve 6 mg of lyophilized recombinant human interferon-α in 300 ul of a solution containing 1% poloxamer 188 and 1.5% (g / ml) sodium alginate to form an inner aqueous phase;
[0050] (3) Preparation of colostrum
[0051] Inject the inner aqueous phase into the organic phase, stir and emulsify for 1 minute at 10,000 rpm to form colostrum (W / O);
[0052] (4) Preparation of double emulsion
[0053] Add colostrum to the mixture containing 2.5% (g / ml) PVA and 3.0% (g / ml) CaCl under the condition of stirring at 1800rpm 2 In the solution (outer water phase 1), emulsify for 10 minutes to form double emulsion (W / O / W); wherein the ratio of colostrum to outer water phase 1 is 1:40;
[0054] (5) Fo...
Embodiment 2
[0061] Example 2 Preparation of Interferon PLGA Composite Microspheres
[0062] (1) Preparation of PLGA solution
[0063] 600 mg PLGA was dissolved in 4 ml dichloromethane to form an organic phase (15% g / ml).
[0064] (2) Preparation of interferon sodium alginate (Sa) solution
[0065] Dissolve 6 mg of lyophilized recombinant human interferon-α in 300 ul of a solution containing 1% poloxamer 407 and 0.8% (g / ml) sodium alginate to form an inner aqueous phase;
[0066] (3) Preparation of colostrum
[0067] Inject the inner aqueous phase into the organic phase, stir and emulsify at 5000rpm for 10 minutes to form colostrum (W / O);
[0068] (4) Preparation of double emulsion
[0069] Add colostrum to the mixture containing 2.5% (g / ml) PVA and 1.5% (g / ml) CaCl under the condition of stirring at 1600rpm 2 In the solution (outer water phase 1), emulsify for 10 minutes to form double emulsion (W / O / W); wherein the ratio of colostrum to outer water phase 1 is 1:60;
[0070] (5) Form...
Embodiment 3
[0076] Example 3 Preparation of Interferon PLGA Composite Microspheres
[0077] (1) Preparation of PLGA solution
[0078] 800 mg PLGA was dissolved in 4 ml dichloromethane to form the organic phase (20% g / ml).
[0079] (2) Preparation of interferon sodium alginate solution
[0080] Dissolve 6 mg of lyophilized recombinant human interferon-α in 300 ul of a solution containing 1% poloxamer 188 and 0.5% (g / ml) sodium alginate to form an inner aqueous phase;
[0081] (3) Preparation of colostrum
[0082] Inject the above-mentioned internal aqueous phase into the above-mentioned organic phase, stir and emulsify at 18000rpm for 1 minute to form colostrum (W / O);
[0083] (4) Preparation of double emulsion
[0084] Add colostrum to the mixture containing 2.5% (g / ml) PVA and 1.5% (g / ml) CaCl under the condition of stirring at 2500rpm 2 In the solution (outer water phase 1), emulsify for 15 minutes to form double emulsion (W / O / W); wherein the ratio of colostrum to outer water phase...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com