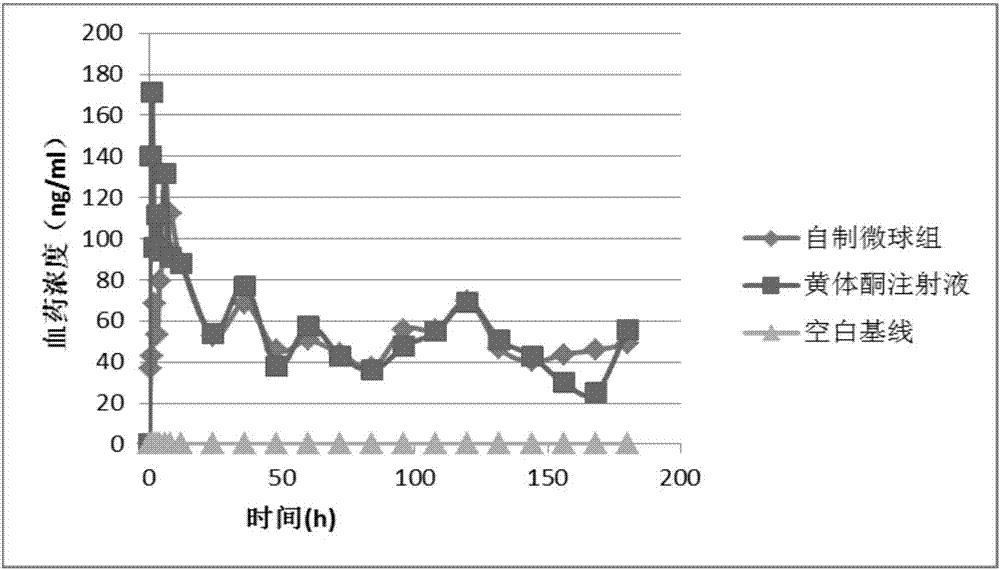
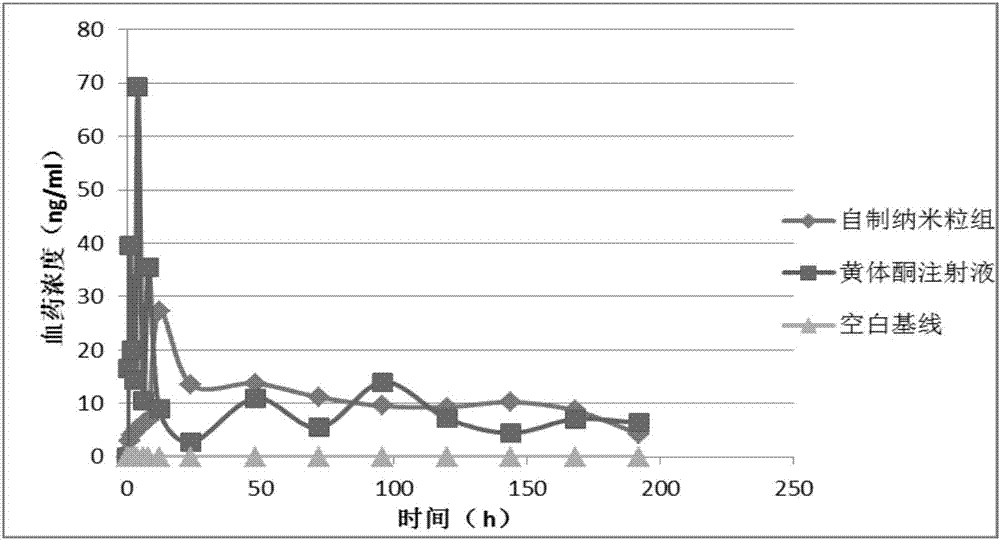
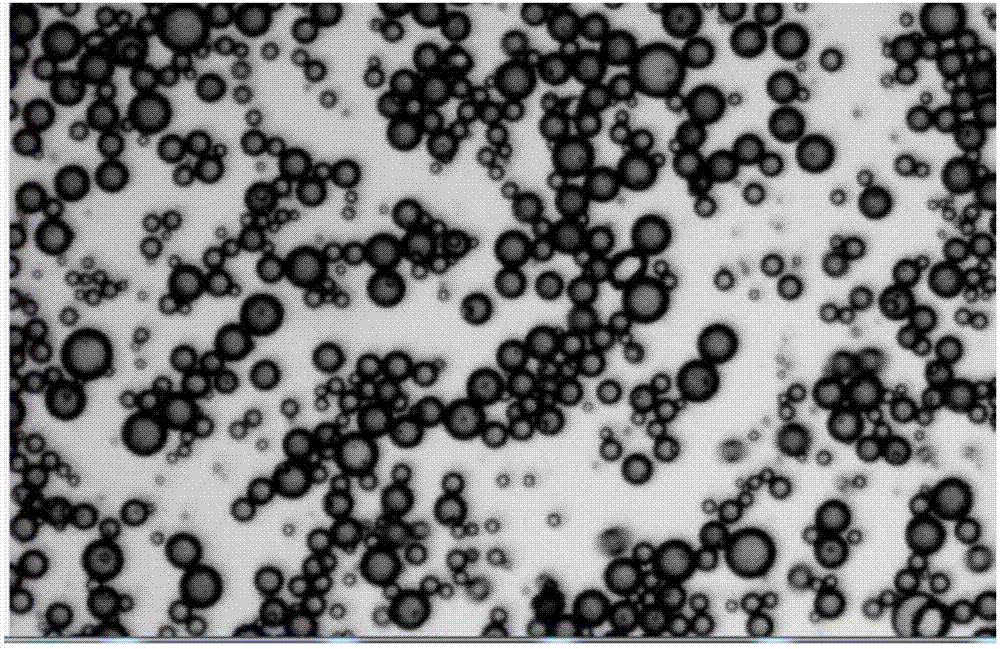
Progestin sustained-release microsphere and nanoparticle and preparation method thereof and progestin sustained-release injection
A technology for slow-release microspheres and progesterone, which can be used in pharmaceutical formulations, microcapsules, nanocapsules, etc., and can solve problems such as pain, inconvenience of progesterone injection, and induration at the injection site.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The preparation of embodiment 1 progesterone microspheres
[0067] Weigh 150mg PLGA (50 / 50 (the molar ratio of lactic acid and glycolic acid), molecular weight 13000 Daltons, the following examples represent the same as Example 1), 75mg progesterone, and dissolve in 1.5mL methylene chloride to form an organic phase. The organic phase was quickly poured into 25 mL of 1% PVA (Kuraray Japan) solution, and the o / w emulsion was formed by high-speed shearing at 12000 rpm for 2 min. After diluting with 5 times the volume of water, distill under reduced pressure at 40°C for 15 minutes to volatilize the organic solvent to solidify the microspheres. After the solidified microspheres are washed with deionized water to excess PVA, the microspheres are collected by centrifugation and freeze-dried to obtain progesterone controlled-release microspheres for intramuscular injection.
[0068] Among them, the freeze-drying process includes: pre-freezing at -40°C for four hours, vacuuming...
Embodiment 2-4
[0072] Embodiment 2-4 Different molecular weight PLGA prepares microsphere
[0073] Weigh 150mg of PLGA (50 / 50) with molecular weights of 13,000, 25,000, and 30,000 Daltons; 75mg of progesterone were dissolved in 1.5mL of dichloromethane to form an organic phase. The organic phase was quickly poured into 25 mL of 1% PVA solution, and was sheared at 12000 rpm for 2 min to form an o / w emulsion. After diluting with 5 times the volume of water, distill under reduced pressure at 40°C for 15 minutes to volatilize the organic solvent to solidify the microspheres. After the solidified microspheres are washed with deionized water to excess PVA, the microspheres are collected by centrifugation and freeze-dried to obtain progesterone controlled-release microspheres for intramuscular injection. The prescription among the embodiment 2-4 is referring to table 2:
[0074] The prescription in the embodiment 2-4 of table 2
[0075]
Embodiment 5-7
[0076] Example 5-7 Preparation of microspheres with different theoretical drug loads
[0077] Weigh 150mg of PLGA (50 / 50; 13000), weigh 75mg, 90mg, and 100mg of progesterone, and dissolve in 1.5mL of dichloromethane to form an organic phase. The organic phase was quickly poured into 25 mL of 1% PVA solution, and was sheared at 12000 rpm for 2 min to form an o / w emulsion. After diluting with 5 times the volume of water, distill under reduced pressure at 40°C for 15 minutes to volatilize the organic solvent to solidify the microspheres. After the solidified microspheres are washed with deionized water to excess PVA, the microspheres are collected by centrifugation and freeze-dried to obtain progesterone controlled-release microspheres for intramuscular injection. See Table 3 for the theoretical drug loading of the progesterone microspheres prepared in Examples 5-7:
[0078] Theoretical drug loading of the progesterone microspheres prepared by table 3 embodiment 5-7
[0079] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com