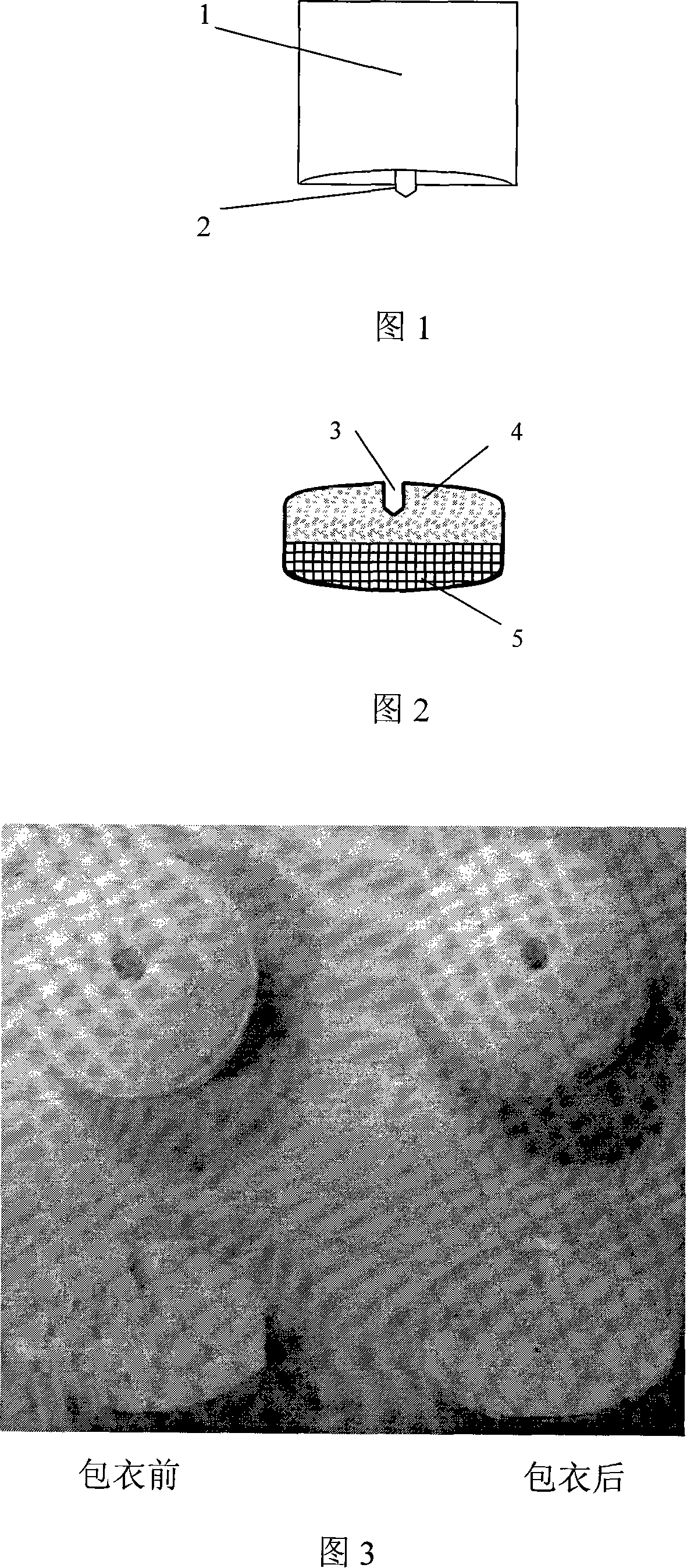
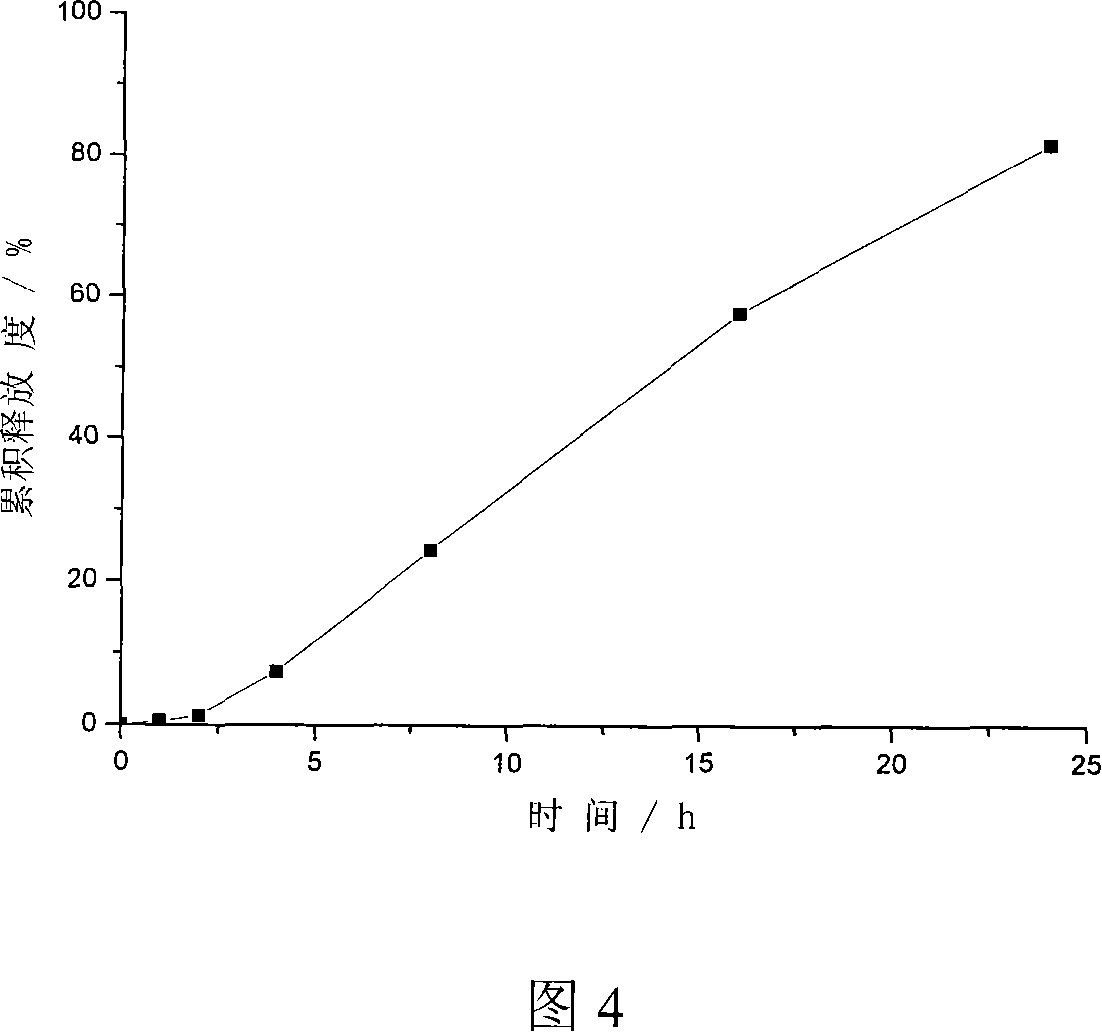
Method of producing double-layer core permeation pump patch of medicament
A technology of osmotic pump tablets and drug layers, which is applied in the direction of medical formula, medical preparations of non-active ingredients, pill delivery, etc., can solve the problems that the punching speed needs to be improved, maintain blood drug concentration, simplify the process, overcome The effect of taking more
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] A preparation method of a double-layer core osmotic pump tablet comprises the following steps:
[0026] 1) Mix 1-100 parts by weight of drug or drug solid dispersion with 1-150 parts by weight of penetrant, 1-150 parts by weight of thickener and 0.1-200 parts by weight of filler passed through a 100-mesh sieve, and add 0.1- 50 parts by weight of binder are wet granulated, dried in an oven at 55-65 degrees, granulated, and 0.1-30 parts by weight of lubricant is added to obtain the core material of the drug layer. The weight percentage of the drug in the drug solid dispersion is 10-70%;
[0027] 2) Mix 1-150 parts by weight of penetrating agent, 1-150 parts by weight of expansion agent, and 0.1-200 parts by weight of filler that have passed through a 100-mesh sieve, and add 0.1-50 parts by weight of binder to wet granulate. Dry at 55-65 degrees, granulate, add 0.1-30 parts by weight of lubricant to obtain the core material of the push layer, and use water or alcohol as t...
Embodiment 1
[0041] Mix insoluble drug nifedipine 30g with sodium chloride 60g passing through a 100-mesh sieve, polyvinylpyrrolidone 80g, and starch 8g, and then make a soft material with an aqueous solution of 10% by weight of polyvinylpyrrolidone and pass through a 16-mesh sieve. The granules are put into an oven and dried for 24 hours at 60 degrees, then granulated through a 14-mesh sieve, and 1% by weight of magnesium stearate is added to obtain the drug layer tablet core material. Mix 50 g of carboxymethylcellulose sodium salt, 50 g of sodium chloride, and 6 g of starch that have passed through a 100-mesh sieve, and then use an aqueous solution of 10% by weight polyvinylpyrrolidone to make a soft material, pass through a 16-mesh sieve for granulation, and put into an oven Dried at 60°C for 24 hours, granulated through a 14-mesh sieve, and added with 1% by weight magnesium stearate to obtain the core material for the push layer. Use a tablet press with a steel needle on the upper punc...
Embodiment 2
[0044] The nifedipine-poloxamer 188 solid dispersion (nifedipine weight [0] percentage is 50%) 60g that contains insoluble drug nifedipine and the sodium chloride 40g that crosses 100 mesh sieves, polyoxyethylene pyrrolidone After mixing 50g and 10g of microcrystalline cellulose evenly, make a soft material with an aqueous solution of 10% by weight of polyvinylpyrrolidone, pass through a 16-mesh sieve to granulate, put it into an oven at 60 degrees and dry it for 24 hours, and then pass through a 14-mesh sieve The granules are granulated, and 1% by weight of magnesium stearate is added to obtain the drug layer tablet core material. After the carboxymethylcellulose sodium salt 50g that crossed 100 mesh sieves, sodium chloride 50g, and microcrystalline cellulose 40g were mixed evenly, soft materials were made with an aqueous solution of 10% by weight polyvinylpyrrolidone, and granulated through 16 mesh sieves. Put it into an oven and dry it for 24 hours at 60 degrees, then pass ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com