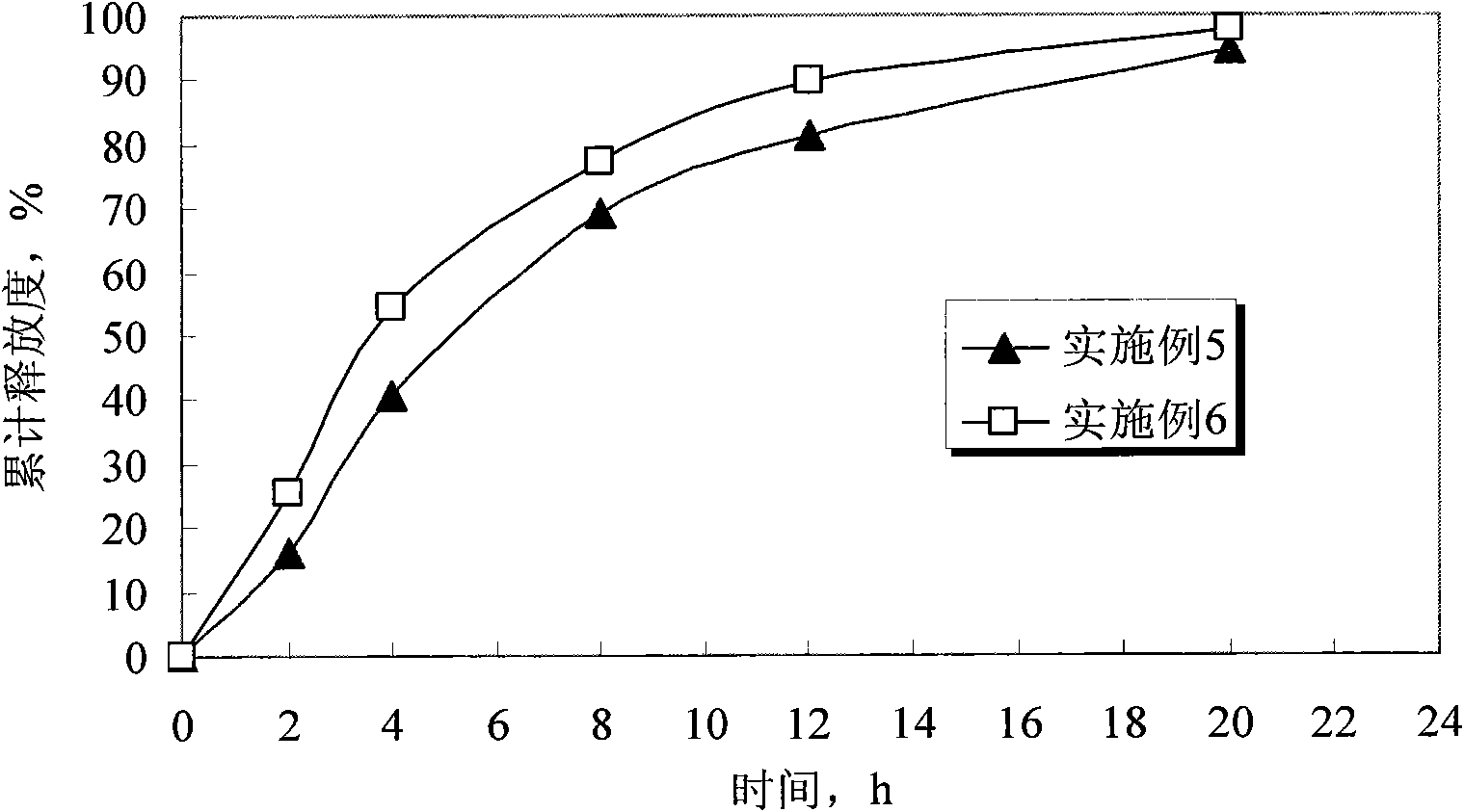
Venlafaxine hydrochloride controlled release tablets and preparation method thereof
A technology of venlafaxine hydrochloride and controlled-release tablets, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve problems such as low drug load and avoid difficulty in swallowing , The effect of reducing production cost and reliable quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
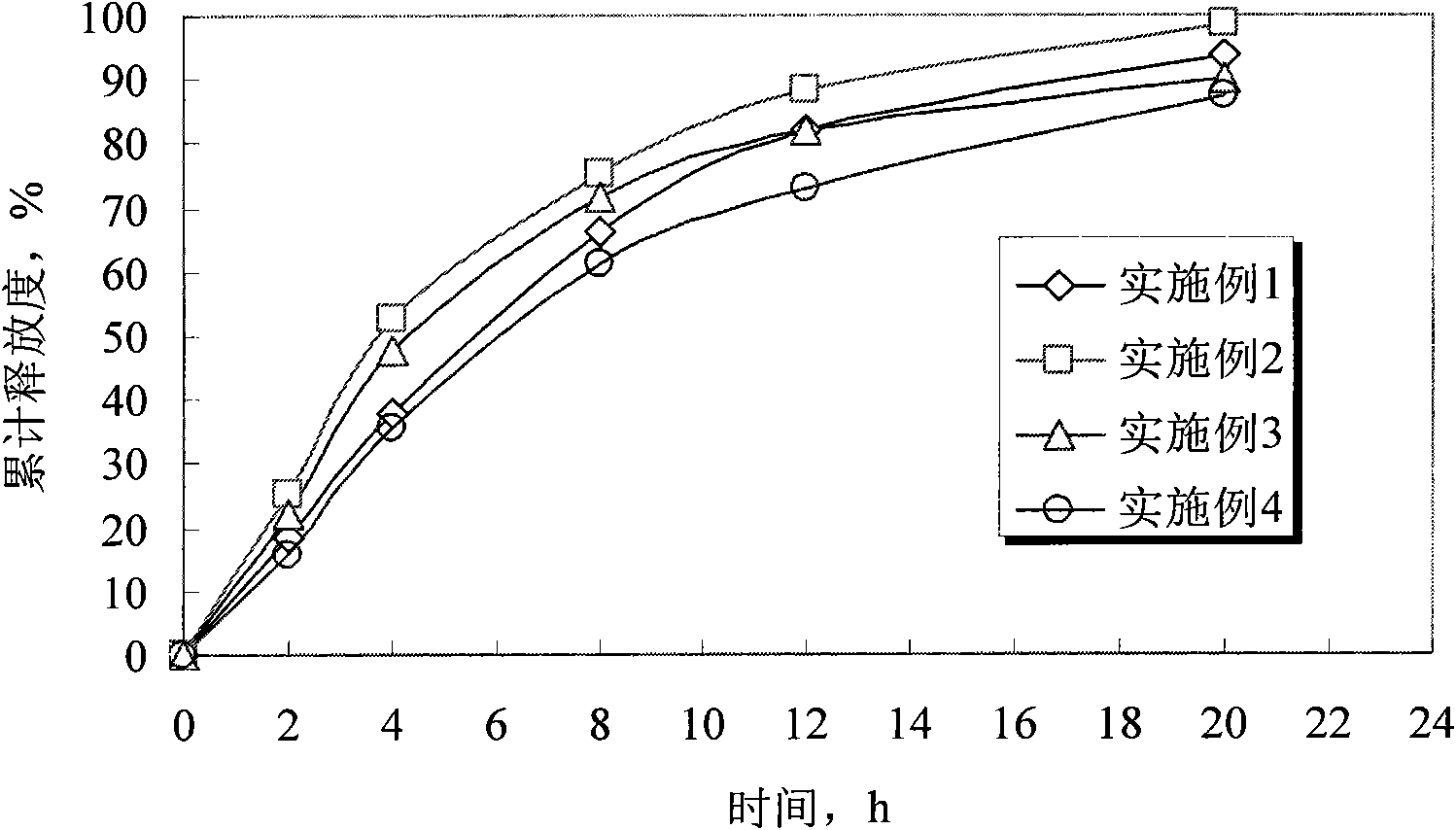
Embodiment 1
[0054] (1) Tablet core prescription
[0055] Composition g / 1000 tablets
[0056] Venlafaxine Hydrochloride 85
[0057] Polyethylene oxide (molecular weight 5 million) 5
[0058] Microcrystalline Cellulose 40
[0060] Dextrin 20
[0061] Povidone 5
[0063] Theoretical weight per tablet: 165.5mg
[0064] (2) Preparation method
[0065] Mix the proportioned venlafaxine hydrochloride, polyoxyethylene, microcrystalline cellulose, sodium chloride, dextrin, and povidone evenly, add an appropriate amount of ethanol to make a soft material, granulate with a 30-mesh sieve, and dry at 40°C. 20 mesh whole grains. Add the proportioned amount of magnesium stearate, mix well, and press into tablets with a shallow concave die to obtain the product.
[0066] The composition ratio of the controlled-release film coat is as follows:
[0067] Cellulose acetate 100g
[0068] Dimethyl phthalate 1g
[0069] Macrogol 1500 20g
...
Embodiment 2
[0075] (1) Tablet core prescription
[0076] Composition g / 1000 tablets
[0077] Venlafaxine Hydrochloride 85
[0078] Polyethylene oxide (molecular weight 5 million) 10
[0079] Microcrystalline Cellulose 10
[0080] Sodium chloride 20
[0081] Dextrin 25
[0082] povidone 5
[0084] Theoretical weight per tablet: 155.5mg
[0085] (2) The preparation method is the same as in Example 1.
[0086] Embodiment 2 selects the diameter as The weight gain of the controlled-release film coating is controlled in the range of about 5%; double-sided laser drilling is adopted, and the pore size is controlled in the range of about 0.3 mm to 0.4 mm.
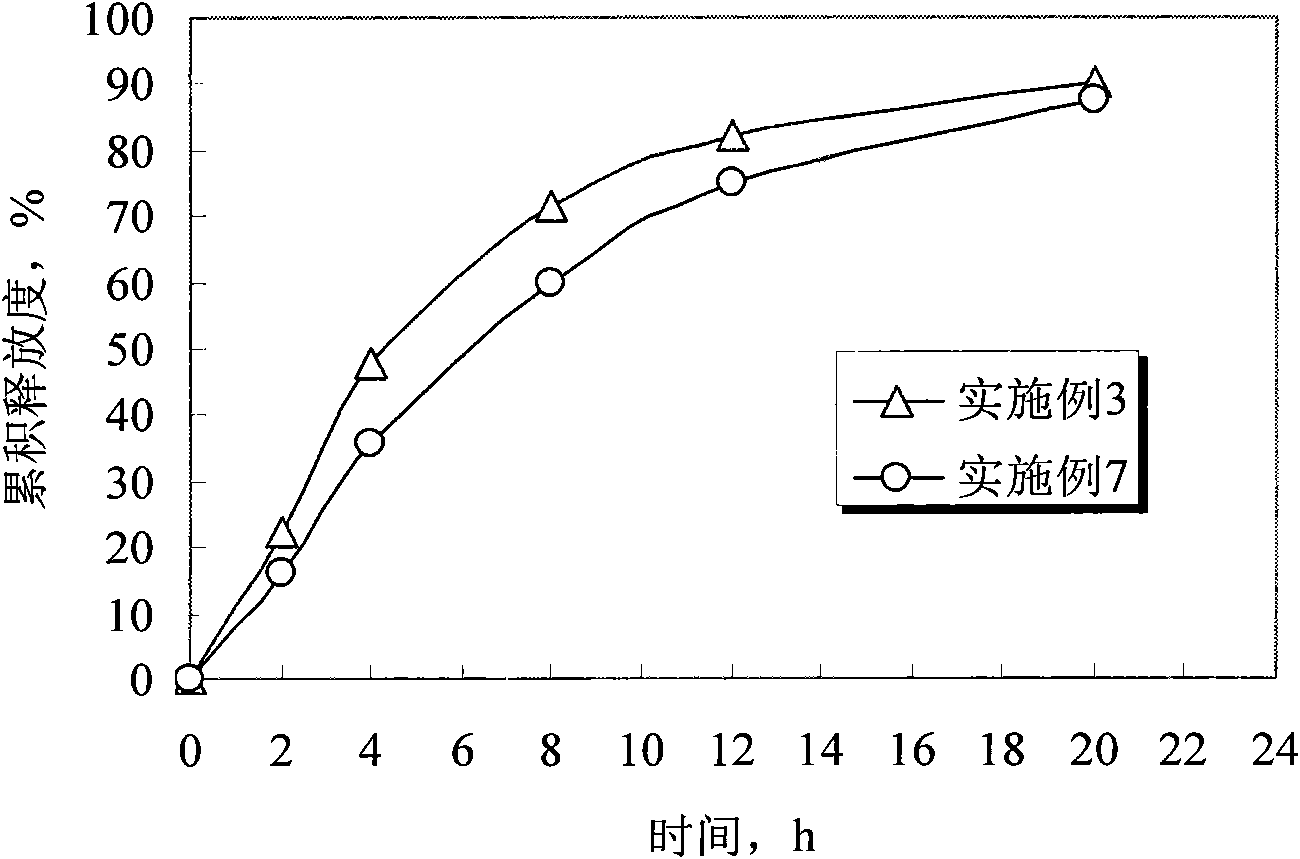
Embodiment 3
[0088] (1) Tablet core prescription
[0089] Composition g / 1000 tablets
[0090] Venlafaxine Hydrochloride 85
[0091] Polyethylene oxide (molecular weight 5 million) 5
[0092] Microcrystalline Cellulose 35
[0093] Sodium chloride 15
[0094] Dextrin 54
[0095] Povidone 10
[0097] Theoretical weight per tablet: 205mg
[0098] (2) preparation method is the same as embodiment 1
[0099] Embodiment 3 selects diameter as The weight gain of the controlled-release film coating is controlled in the range of about 4%; the single-sided laser drilling is adopted, and the pore size is controlled at about 0.5mm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com